 |
 |
| Anim Biosci > Volume 37(1); 2024 > Article |
|
Abstract
Objective
Mitragyna speciosa Korth is traditionally used in Thailand. They have a high level of antioxidant capacities and bioactive compounds, the potential to modulate rumen fermentation and decrease methane production. The aim of the study was to investigate the different levels of microencapsulated-Mitragyna leaves extracts (MMLE) supplementation on nutrient degradability, rumen ecology, microbial dynamics, and methane production in an in vitro study.
Methods
A completely randomized design was used to assign the experimental treatments, MMLE was supplemented at 0%, 4%, 6%, and 8% of the total dry matter (DM) substrate.
Results
The addition of MMLE significantly increased in vitro dry matter degradability both at 12, 24, and 48 h, while ammonia-nitrogen (NH3-N) concentration was improved with MMLE supplementation. The MMLE had the greatest propionate and total volatile fatty acid production when added with 6% of total DM substrate, while decreased the methane production (12, 24, and 48 h). Furthermore, the microbial population of cellulolytic bacteria and Butyrivibrio fibrisolvens were increased, whilst Methanobacteriales was decreased with MMLE feeding.
Tropical plants are rich in bioactive compounds (BC) namely phenolic, flavonoid compounds, and antioxidant capacities [1,2], which may have anti-microbial effects, especially in methanogen and protozoal populations, and which improve the characteristics of rumen fermentation [3,4]. The BC have been demonstrated to influence product quality and health condition that play a vital role in animal nutrition [5].
One of the alternative sources of plants containing BC is Mitragyna speciosa Korth, a tropical plant in Southeast Asia including Myanmar, Malaysia, and Thailand [6]. M. speciosa is popularly known as Kratom in Thailand. They are traditionally used to treat tiredness, opioid addiction, and relieve pain [7]. The leaves of M. speciosa have shown the presence of BC such as flavonoids, alkaloids, glycoside, and triterpenoids [8]. This plant has been demonstrated to have several pharmacological properties such as antibacterial, anti-inflammatory, and antioxidant [9]. Accordingly, Phesatcha et al [10] reported that the Mitragyna leaf powder supplementation as a BC can improve rumen fermentation, whilst decrease rumen protozoa, and methane production. Chanjula et al [11] revealed that Mitragyna leaf powder enhanced rumen ecology by increasing nutrient digestibility, volatile fatty acid (VFA, propionic acid profile), and reducing methane production in goats.
Microencapsulation is an emerging technology that is commonly used nowadays in animal nutrition for the preparation of stable products (vitamins, minerals, fatty acids, as well as BC) [12]. This technique can act as a physical barrier to protect pharmaceuticals from harsh external environment, which increases the stability of the substance [13]. Among microencapsulation procedures, spray-drying is a practical approach that could produce a constant microcapsule [14]. However, no previous research has evaluated protection of BC by microencapsulation technique of Mitragyna leaves as a strategy to enhance their interactions with ruminal fermentation. Therefore, this study aimed at testing the susceptibility of microencapsulated BC from Mitragyna leaves to in vitro nutrient degradation, rumen ecology, and microbial diversity.
The collection of rumen fluid from Thai-crossbred dairy cows was permitted by the Institute of Animals for Scientific Purpose Development (IAD), Thailand (number U1-06878-2560).
The plant sources were harvested at Rajamangala University of Technology Srivijaya (MUTSV), Nakhon Si Thammarat, Thailand. Fresh Mitragyna leaf was dried at 60┬░C. The dried Mitragyna was ground through a sieve opening of 1 mm (Cyclotech Mill, Tecator, Hoganas, Sweden). The powder was mixed with water and heated in a Microwave to 60┬░C, and after 35 minutes the particulates filtered out. The liquids were combined with tween 80 and chitosan [15], and they were spray-dried microencapsulated-Mitragyna leaves extracts (MMLE) by using BŪÜchi B-191 Mini Spray Dryer [16]. The surface morphology of MMLE was observed using a field-emission scanning electron microscope (FE-SEM; model: Mira, Tescan Co., Brno, Czech Republic) according to Ko et al [17]. MMLE were chemically analyzed for dry matter (DM; number 967.03), ash (number 942.05), and crude protein (CP; number 984.13) following the methods of AOAC [18], as shown in number 973.18. Fiber fractions (neutral-detergent fiber [NDF] and acid-detergent fiber [ADF]) were determined using Ankom A200i Fibre Analyser (Ankom Technology Co., New York, USA); according to Van Soest et al [19]. MMLE were analyzed for BC especially total phenolic compound (TPC) using FolinŌĆōCiocalteu reagent by absorbance at 765 nm [20] and total flavonoid compound (TFC) following the method of Top├¦u et al [21], based on colorimetric changes with a 10% aluminum chloride solution read at 415 nm. Moreover, the sample was analyzed the antioxidant capacities including 2,2-diphenyl-1-picrylhydrazyl (DPPH) [22], 2, 2ŌĆ▓-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) [23], and ferric reducing antioxidant power (FRAP) [24], which are additional explained in Phupaboon et al [25].
The study was assigned in a completely randomized design (CRD). Total dietary substrates (the ratio of rice straw to concentrate at 60:40) were weighed at 0.5 g into the 60 mL bottles, then the treatments were supplemented with MMLE at 0%, 4%, 6%, and 8% of total DM substrate, respectively.
The rumen fluid donors were four Thai-crossbred dairy cows (body weight, 400┬▒10 kg). The animals consumed total mixed ration twice daily at 7:00 and 16:00 oŌĆÖclock, and they had unlimited access to mineral block and clean water for at least 14 days following the National Research Council (NRC) [26] requirement for dairy cows. Samples of the rumen fluid were taken using a tube connected with a vacuum pump set through the mouth to the middle of the rumen and into a plastic flask. The samples were transferred into a bottle with thermal insulation at 39┬░C after being filtered through four layers of folded cheesecloth. Part of the preparation of the medium solution (2,000 mL) contains 0.24 mL of micro-mineral solution, 2.44 mL of resazurine, 99.0 mL of reduction solution, 480.0 mL of macro-mineral solution, 480.0 mL of buffer solution, and 950.0 mL of distilled water, respectively. Under constant CO2 flushing, rumen fluid was combined with the medium substrate at 1:2 (mL/mL). Substrates in total (concentrate and roughage sources) were weighed into glass bottles (60 mL), then the respective treatments, MMLE was added at 0.00, 0.02, 0.03, and 0.04 g DM. The bottles were capped with rubber stoppers and aluminum caps. Rumen inocula mixture was added (40 mL) to the bottles and incubated at 39┬░C, as described in Matra et al [27].
During incubation, the production of gas was recorded at 1, 2, 4, 6, 8, 12, 24, 48, 72, and 96 h (3 bottles/treatment). The equation of ├śrskov and McDonald [28] was used to analyze all gas production data; Y = a+b (1ŌłÆeŌłÆct), where Y = gas generated at time ŌĆ£tŌĆØ (mL), a = the gas production from the immediately soluble fraction (mL), b = the gas production from the insoluble fraction (mL), c = the gas production rate constant for the insoluble fraction (mL/h), and t = incubation time (h). The samples were collected separately for pH, microbial population, ammonia nitrogen (NH3-N), and VFA analyses at 12, 24, and 48 h-after incubation (2 bottles/treatment). A portable pH meter was used to determine the pH (HANNA Instruments HI 8424 microcomputer, Singapore). The rumen fluid instances were centrifuged at 16,000├Śg for 15 minutes after being filtered through instances cheesecloth, then to analyze the NH3-N concentration using micro-Kjeldahl techniques, the supernatant was kept at ŌłÆ20┬░C [18] and VFA profiles (HPLC; ETL Testing Laboratory, Inc., Cortland, NY, USA); according to Samuel et al [29]. Additionally, in vitro nutrient degradability was measured using a different set (2 bottles/treatment). The production of methane (CH4; 3 bottles/treatment) was measured using GC machine (GC-2014; Shimadzu Co Ltd., Kyoto, Japan); methane production (% v/v) = (Peak area/18,108)/0.3, where 0.3 = the volume of gas was kept in the bottle (10 mL) and 18,108 = the slope estimates of the standard methane graph.
Approximately, 1 mL of rumen fluid from in vitro study was extracted for total genomic DNA (gDNA) following to the method of QIAamp Fast DNA Stool Mini kit (Qiagen, Hilden, Germany). The gDNA quality (the concentration at Ōēź50 ng/╬╝L) was indicated by absorbance at OD260/280 = 1.8 to 2.0 using Nanodrop spectrophotometer (Thermo Scientific, USA). The microbial population including Ruminococcus albus, Ruminococcus flavefaciens, Fibrobactor succinnogenes, Butyribrivio fibrisolvens, Megasphaera elsdenii, and Methanobacteriales were identified using the specific primers through real-time polymerase chain reaction (PCR) technique, as shown in Table 1. The real-time PCR amplification and detection were performed by Maxima SYBR Green qPCR Master Mix using Chromo 4TM system (Bio-Rad, Hercules, CA, USA), more detail of the protocols was demonstrated in Koike and Kobayashi [30].
The data were analyzed using the general linear model procedure following to the method of SAS [34], for a CRD; Yij = ╬╝+Žäi+╔øij, where ╬╝ = overall mean, Žäi = treatment effect, ╔øij = residual error, and Yij = observation. The mean values of the experimental treatments were compared with TukeyŌĆÖs test. Differences between treatment means were reported as statistically different had p-values of <0.05 and <0.01. Trends of MMLE supplementation responses were analyzed by Orthogonal polynomials.
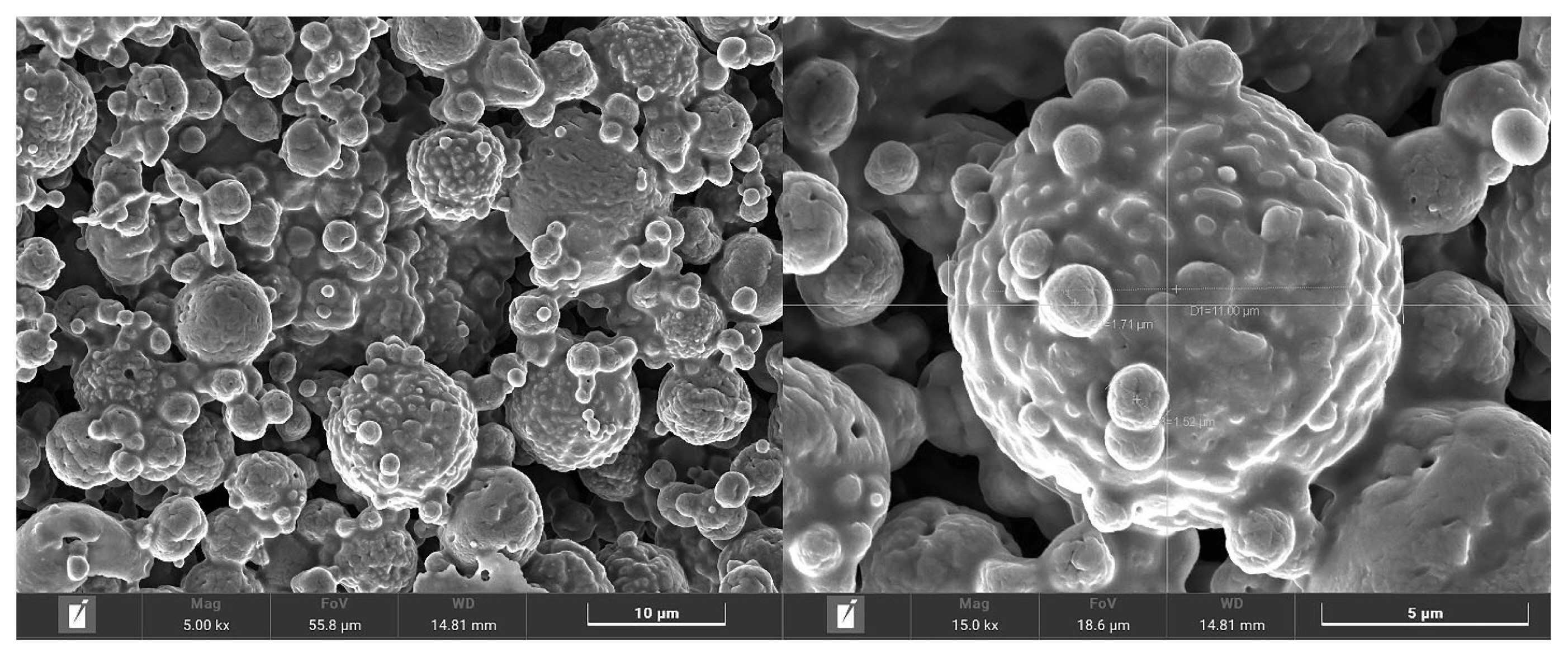
The nutritive values of MMLE were 90.1% DM, and 96.4%, 18.6%, 72.2%, and 21.9% DM basis for OM, CP, NDF, and ADF, respectively. Importantly, BC contained in MMLE were 307.8 mg gallic acid equivalent/g DM of TPC and 105.3 mg quercetin equivalent/g DM of TFC. In the antioxidant capacity, including 94.8% DPPH, 90.3% ABTS, and 34.4 mg trolox equivalent/g DM of FRAP), as shown in Table 2. Moreover, morphological characterization of MMLE, chitosan microparticles showed that they had entirely spherical surface morphologies, with porous surrounding particle spheres interspersed with smooth and rough surfaces. The MMLE identified numerous particles with sizes ranging from 1.5 to 11.0 ╬╝m in diameter (Figure 1).
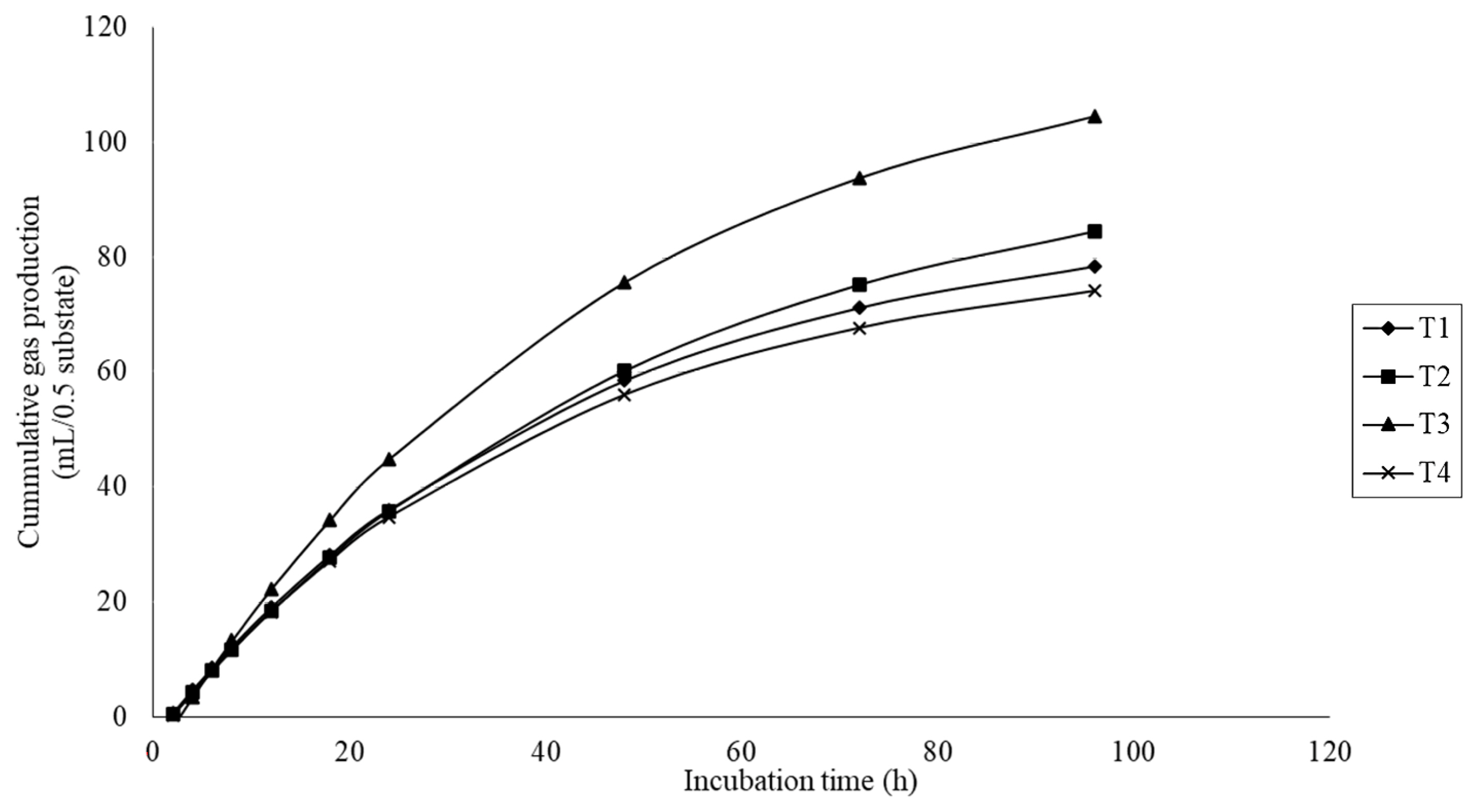
The gas production results are presented in Table 3. Gas production kinetics, including the gas production from the immediately soluble fraction (a), the potential extent of gas production (a+b), and the gas production from the insoluble fraction (b) were significantly different (quadratic effect; p<0.01) with MMLE supplementation. There was a significant difference (p<0.05) on the gas production rate constant for the insoluble fraction (c), with higher values for the treatment fed 6% MMLE. In addition, the cumulative gas production was quadratically increased (p<0.01) with MMLE addition (Figure 2).
The MMLE had the greatest in vitro dry matter degradability (IVDMD) (p<0.05) at 12, 24, and 48 h of fermentation (quadratic effect) when supplemented with 6% of total DM substrate. The lowest IVDMD occurred with MMLE addition at 8% of total DM substrate. Furthermore, this parameter was not linearly influenced, as presented in Table 3.
Table 4, the ruminal pH (12, 24, and 48 h) were not affected (p>0.05), when increasing the level of MMLE. The ammonia nitrogen content (24 and 48 h) was quadratically increased (p<0.05 and p<0.01) when MMLE was added at 6% of total DM substrate. This concentration at 24 and 48 h was significantly higher (p<0.05 and p<0.01) than at 12 h by the supplementation of MMLE.
The 6% MMLE had significantly (quadratic effect; p<0.05) greater acetate, propionate, acetate to propionate ratio, and total VFA production, and it had the highest propionate content (p<0.05) when compared with the control treatments. The content of butyrate did not differ (p>0.05) with MMLE addition. Moreover, methane production (after 12, 24, and 48 h of fermentation) was linearly decreased (p<0.05) when MMLE level was increased. The supplementation of 6% MMLE had the lowest methane content (p<0.05) compared with other treatments, as shown in Table 5.
Based on species level, MMLE supplementation was able to change the bacterial and archaeal population. The present findings showed that MMLE supplementation increased cellulolytic bacteria, namely Ruminococcus albus (p<0.05), Ruminococcus flavefaciens (p<0.05), and Fibrobactor succinnogenes (p<0.05). The relative abundance of Butyribrivio fibrisolvens increased (p<0.05) in 6% MMLE compared with control, while Megasphaera elsdenii was not statistically different (p>0.05) among treatments. Importantly, Methanobacteriales was linearly decreased (p<0.05) when fed the MMLE (Table 6).
In the present study, gas kinetics, especially the gas production rate constant for the insoluble fraction (c), were improved by the MMLE supplementation. It could be due to the capability of BC to enhance microbial growth and activity and its ability to bind in the contents of protein and fiber [3,35]. Accordingly, Phesatcha et al [10] stated that Mitragyna leaves powder enhanced gas production kinetics, itŌĆÖs possible that it improved the rumen microbe and increased the substrateŌĆÖs capacity to degrade, so improving the kinetics of gas production.
The MMLE supplementation to the diet clearly increased IVDMD, which was significantly higher with the addition of 6% of total DM substrate. This might be explained by an increase in the number of microbes, which would cause more feed to breakdown, which was one important role of the BC contained in MMLE. Zhan et al [36] explained that flavonoids and phenolics have a range of biological effects that can impact ruminal microbes, which in turn increases how feed is degraded in the rumen. Sommai et al [37] reported that flavonoid extracts from Alternanthera sissoo supplementation significantly increased in vitro degradability.
This research has shown that ruminal pH did not influence the treatments. For typical rumen fermentation, microbial growth, and microbial activity, the data were in the normal range (6.85 to 6.99). Accordingly, Wanapat [38] reported that pH ranges between 6.5 and 7.0 are optimum for microbial activity and growth. Strategic addition of phenolic-containing feedstuffs can enhance rumen fermentation by preserving a higher pH [39]. Furthermore, MMLE supplementation was improved NH3-N concentration both 12, 24, and 48 h. This may be a result of the plant-bioactive extractŌĆÖs potential to enhance the proteolysis process. Plant-based bioactive supplementation increases the concentration of ruminal NH3-N, which was confirmed by Ahmed et al [40]. Furthermore, it could be a positive effect of the concentrate and MMLE containing protein source at 14.6% and 18.6% CP, thus increasing the amount of NH3-N present as a result.
Under this investigation, MMLE supplementation increased the molarity of VFAs especially propionate and total VFA production, while decreased acetate production. Patra and Saxena [41] explained that BC may also cause a change in propionate produced when there is an excess of hydrogen. Hydrogen is used to create propionate instead of being the major substrate for the methane production pathway [42]. These findings agree with Totakul et al [43] who revealed that the Cnidoscolus leaves pellet supplementation significantly increased propionate concentration, while decreased acetate to propionate ratio. Propionate content typically increases when rumen methanogenesis is inhibited, and this was also shown in the current investigation. Bodas et al [44] demonstrated that phenolic acids and polyphenols suppress methanogenesis, while also improving fermentation parameters. Therefore, phenolics and flavonoids from multi-functional tropical plants have the potential to directly inhibit methanogen population and activity. Furthermore, BC in feeds has been demonstrated, whether in natural form or as plant extracts, to have an impact on the rumenŌĆÖs ability to reduce methane production by rumen microorganisms. Cellulolytic bacteria are among the specific microorganisms that BC directly affects. It causes F. succinogenes (the non-hydrogen producing bacteria) to produce more propionate and reduce the acetate to propionate ratio [45]. In this study, MMLE supplementation clearly decreased methane production. As described in Chanjula et al [11], dried Mitragyna leaves linearly decreased methane production and F. succinogenes quadratically increased when the level of Mitragyna leaves was added. Huang et al [46] showed that Paulownia hybrid leaves decreased methane production, it could be the result of a decline in Archaea especially methanogens due to secondary metabolite activities.
In the current study, the cellulolytic bacteria population increased with the levels of MMLE supplementation. Consequently, the phenolic and flavonoid containing in MMLE, compounds could influence the cellulolytic bacteria activities especially when MMLE was supplemented at 8% of total DM substrate. BC activity alters protein translocation, phosphorylation processes, ion gradients, electron transport, and other enzyme-dependent processes, which results in the impacted cellulolytic bacteria losing chemiosmotic control [47]. Nevertheless, BC should be supplemented at a suitable level for microbe activity, especially cellulolytic bacteria. F. succinogenes, R. albus, and R. flavefaciens have been identified as the main cellulolytic bacterial species in the rumen and more these groups could improve ruminant degradation of fiber [48]. According to Chanjula et al [11] stated that dried Mitragyna leaves was enhanced F. succinogenes, R. albus, and R. flavefaciens. Huang et al [46] revealed that Paulownia hybrid leaves containing flavonoid and phenolic compounds increases total bacteria, as well as in particular species of B. fibrisolvens and F. succinogenes. This may be explained by the ruminal microbesŌĆÖ response to the flavonoids and phenolics, perhaps as a result of hydrogenation, which transforms toxic compounds into less toxic forms [49]. Moreover, MMLE addition increased Butyrivibrio fibrisolvens group, while reduced methanogens group (Methanobacteriales), which could be attributed to the availability of BC in the MMLE. Similarly, Phesatcha et al [10] showed that the supplementation of Mitragyna leaves reduced ruminal methanogens population and methane production. BC has an immediate impact on rumen methanogens, by interacting with the proteinaceous adhesin, suppressing methanogen growth, reducing interspecies hydrogen transfer, and inhibiting the methanogen-protozoa complexŌĆÖs formation [50].
Based on the findings, supplementation of MMLE at 6% of total DM substrate enhanced rumen nutrient degradability, fermentation end-products especially propionate production, and decreased methanogens and methane production. Hence, MMLE could be an effective dietary BC and could have the potential to be used for ruminant feed additives.
Notes
ACKNOWLEDGMENTS
The authors wish to acknowledge their appreciation to the Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Thailand.
Figure┬Ā2
Effect of microencapsulated-Mitragyna leaves extracts (MMLE) on cumulative gas production curves after 1ŌĆō96 h of incubation. The treatments (T1ŌĆōT4) were added with MMLE at 0%, 4%, 6%, and 8% of total dry matter substrate, respectively.
Table┬Ā1
The specific primers of rumen microbes
| Species | Specific primers | Primer sequences (5ŌĆ▓-3ŌĆ▓) | PCR products (bp) | References |
|---|---|---|---|---|
| Fibrobacter succinogenes | Fs219f | GGTATGGGATGAGCTTGC | 446 | Koike and Kobayashi [30] |
| Fs654r | GCCTGCCCCTGAACTATC | |||
| Ruminococcus albus | Ra1281f | CCCTAAAAGCAGTCTTAGTTCG | 175 | |
| Ra1439r | CCTCCTTGCGGTTAGAACA | |||
| Ruminococcus flavefaciens | Rf154f | TCTGGAAACGGATGG TA | 295 | |
| Rf425r | CCTTTAAGACAGGAGTTTACA A | |||
| Megasphaera elsdenii | Mef | GACCGAAACTGCGATGCTAGA | 128 | Ouwerkerk et al [31] |
| Mer | TCCAGAAAGCCGCTTTCGCCACT | |||
| Butyrivibrio fibrisolvens | Bff | CGCATGATGCAGTGTGAAAAGCTC | 625 | Fernando et al [32] |
| Bfr | CCTCCCGACACCTATTATTCATCG | |||
| Methanobacteriales | Mbt857f | GGGCTTGCTTTGGAAACTGTT | 343 | Yu et al [33] |
| Mbt1196r | CCCACCGATGTTCCTCCTAA |
Table┬Ā2
Chemical composition of feed used in the experiment
| Items | Concentrate | Rice straw | MLM | MMLE |
|---|---|---|---|---|
| Ingredients (% as fed) | ||||
| ŌĆāCassava chip | 54.0 | |||
| ŌĆāRice bran meal | 17.0 | |||
| ŌĆāPalm kernel meal | 13.0 | |||
| ŌĆāSoybean meal | 10.5 | |||
| ŌĆāUrea | 2.5 | |||
| ŌĆāSulphur | 1.0 | |||
| ŌĆāSalt | 1.0 | |||
| ŌĆāMineral mixed1) | 1.0 | |||
| Chemical composition | ||||
| ŌĆāDM (%) | 90.5 | 89.4 | 93.1 | 90.1 |
| -------------------------------------------------------------------- % DM -------------------------------------------------------------------- | ||||
| ŌĆāOM | 92.2 | 85.4 | 94.8 | 96.4 |
| ŌĆāCrude protein | 14.6 | 2.4 | 19.7 | 18.6 |
| ŌĆāNeutral-detergent fiber | 20.5 | 78.9 | 48.0 | 72.2 |
| ŌĆāAcid-detergent fiber | 8.2 | 52.6 | 19.6 | 21.9 |
| Phytonutrient compound | ||||
| ŌĆāTPC (mg GAE/g DM) | - | - | 306.9 | 307.8 |
| ŌĆāTFC (mg QUE/g DM) | - | - | 119.2 | 105.3 |
| Antioxidant capacity | ||||
| ŌĆāDPPH (%) | - | - | 91.4 | 94.8 |
| ŌĆāABTS (%) | - | - | 95.3 | 90.3 |
| ŌĆāFRAP (mg TROE/g DM) | - | - | 39.0 | 34.4 |
MLM, Mitragyna leaves meal; MMLE, microencapsulated-Mitragyna leaves extracts; DM, dry matter; OM, organic matter; TPC, total phenolic content; TFC, total flavonoid content; GAE, gallic acid equivalent; QUE, quercetin equivalent; DPPH, 2, 2-diphenyl-1-picrylhydrazyl as DPPH radical scavenging activity; ABTS, 2, 2ŌĆ▓-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) as ABTS radical scavenging activity; FRAP, ferric reducing antioxidant power; TROE, trolox equivalent.
Table┬Ā3
Supplementation of microencapsulated-Mitragyna leaves extracts on gas kinetics and nutrient degradability
| MMLE (% of total substrate) | Gas kinetics1) | Cumulative gas2) at 96 h | IVDMD (% DM) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| a | b | c | a+b | 12 h | 24 h | 48 h | ||
| 0 | ŌłÆ4.1a | 85.4a | 0.025a | 81.3a | 81.2a | 57.9a | 62.8a | 68.8a |
| 4 | ŌłÆ3.6b | 91.1b | 0.027b | 87.6b | 87.5b | 64.1b | 68.9b | 73.6b |
| 6 | ŌłÆ3.6b | 105.5c | 0.034c | 101.9c | 101.6c | 64.5b | 69.1b | 73.9b |
| 8 | ŌłÆ3.9b | 81.8d | 0.022d | 77.9d | 77.8d | 60.2c | 63.1c | 70.8c |
| SEM | 0.32 | 1.43 | 0.02 | 1.56 | 1.73 | 0.85 | 0.87 | 0.91 |
| Orthogonal polynomials | ||||||||
| ŌĆāLinear | 0.13 | 0.05 | 0.11 | 0.13 | 0.09 | 0.17 | 0.19 | 0.60 |
| ŌĆāQuadratic | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | 0.03 | 0.04 | 0.04 |
| ŌĆāCubic | 0.25 | 0.73 | 0.06 | 0.86 | 0.57 | 0.16 | 0.17 | 0.86 |
MMLE, microencapsulated-Mitragyna leaves extracts; IVDMD, in vitro dry matter degradability; SEM, standard error of mean.
Table┬Ā4
Supplementation of microencapsulated-Mitragyna leaves extracts on ruminal pH and ammonia-nitrogen concentration
| MMLE (% of total substrate) | pH | Ammonia nitrogen (mg/dL) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | |
| 0 | 6.96 | 6.90 | 6.89 | 9.7a | 10.5a | 13.1a |
| 4 | 6.94 | 6.94 | 6.92 | 12.0b | 11.7b | 14.9b |
| 6 | 6.96 | 6.95 | 6.91 | 12.6b | 12.8c | 16.8c |
| 8 | 6.99 | 6.95 | 6.94 | 8.7a | 9.5a | 13.3a |
| SEM | 0.01 | 0.01 | 0.02 | 0.25 | 0.23 | 0.46 |
| Orthogonal polynomials | ||||||
| ŌĆāLinear | 0.28 | 0.14 | 0.05 | 0.41 | 0.61 | 0.83 |
| ŌĆāQuadratic | 0.34 | 0.43 | 0.10 | <0.01 | 0.02 | <0.01 |
| ŌĆāCubic | 0.65 | 0.95 | 0.17 | 0.21 | 0.05 | 0.58 |
Table┬Ā5
Supplementation of microencapsulated-Mitragyna leaves extracts on volatile fatty acids and methane production
| MMLE (% of total substrate) | VFA (mol/100 mL) | C2:C3 | Total VFA (mmol/L) | Methane production (%) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| C2 | C3 | C4 | 12 h | 24 h | 48 h | |||
| 0 | 69.4a | 23.7a | 6.9 | 2.95a | 67.4a | 27.5a | 30.8a | 34.9a |
| 4 | 68.5b | 24.9b | 6.6 | 2.75b | 74.1b | 26.6b | 29.9b | 34.0b |
| 6 | 65.0c | 26.9c | 8.1 | 2.40c | 84.8c | 25.1c | 28.4c | 33.5c |
| 8 | 69.2a | 24.5b | 6.3 | 2.85a | 71.7b | 24.9d | 27.6d | 32.3d |
| SEM | 0.46 | 0.37 | 0.25 | 0.08 | 2.25 | 0.07 | 0.05 | 0.05 |
| Orthogonal polynomials | ||||||||
| ŌĆāLinear | 0.42 | 0.53 | 0.88 | 0.50 | 0.37 | 0.02 | 0.01 | 0.01 |
| ŌĆāQuadratic | 0.04 | 0.03 | 0.21 | 0.04 | 0.04 | 0.62 | 0.48 | 0.51 |
| ŌĆāCubic | 0.08 | 0.16 | 0.10 | 0.34 | 0.64 | 0.58 | 0.35 | 0.45 |
Table┬Ā6
Supplementation of microencapsulated-Mitragyna leaves extracts on rumen microbial population
| Species | Incubation time (h) | MMLE (% of total substrate) | SEM | Orthogonal polynomials | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0 | 4 | 6 | 8 | L | Q | C | |||
| Fibrobacter succinogenes (Log copies/mL) | 12 | 5.3a | 5.7b | 5.9c | 5.6b | 0.38 | 0.11 | 0.04 | 0.24 |
| 24 | 5.5a | 6.1b | 6.3c | 6.0b | 0.34 | 0.24 | 0.02 | 0.97 | |
| 48 | 5.7a | 6.2b | 6.5c | 6.0b | 0.26 | 0.62 | 0.01 | 0.69 | |
| Ruminococcus albus (Log copies/mL) | 12 | 7.3a | 7.7b | 8.2c | 7.5b | 0.55 | 0.21 | 0.03 | 0.69 |
| 24 | 7.7a | 7.9a | 8.5b | 7.8a | 0.46 | 0.17 | 0.02 | 0.87 | |
| 48 | 7.9a | 8.1a | 8.8b | 8.0a | 0.62 | 0.52 | 0.04 | 0.13 | |
| Ruminococcus flavefaciens (Log copies/mL) | 12 | 6.6a | 6.8a | 7.3b | 6.7a | 0.53 | 0.57 | 0.03 | 0.57 |
| 24 | 6.8a | 6.9a | 7.6b | 6.8a | 0.44 | 0.24 | 0.03 | 0.94 | |
| 48 | 6.8a | 7.0a | 7.9b | 6.9a | 0.35 | 0.15 | 0.01 | 0.32 | |
| Megasphaera elsdenii (Log copies/mL) | 12 | 7.1 | 7.0 | 7.0 | 7.0 | 1.19 | 0.78 | 0.62 | 0.69 |
| 24 | 7.1 | 7.1 | 7.2 | 7.1 | 1.25 | 0.56 | 0.45 | 0.83 | |
| 48 | 7.0 | 6.8 | 6.8 | 6.8 | 0.88 | 0.11 | 0.51 | 0.49 | |
| Butyrivibrio fibrisolvens (Log copies/mL) | 12 | 6.2 | 6.1 | 6.0 | 6.0 | 1.52 | 0.37 | 0.35 | 0.91 |
| 24 | 6.2 | 6.4 | 6.6 | 6.5 | 0.46 | 0.29 | 0.46 | 0.80 | |
| 48 | 6.2a | 6.3a | 6.8b | 6.4a | 0.34 | 0.28 | 0.01 | 0.88 | |
| Methanobacteriales (Log copies/mL) | 12 | 7.1a | 6.9b | 6.5c | 6.5c | 0.37 | 0.02 | 0.40 | 0.94 |
| 24 | 7.2a | 6.8a | 6.4b | 6.3b | 0.56 | 0.03 | 0.70 | 0.45 | |
| 48 | 7.4a | 6.6b | 5.9c | 5.8c | 0.48 | 0.03 | 0.50 | 0.16 | |
REFERENCES
1. Kholif AE, Gouda GA, Abu Elella AA, Patra AK. Replacing the concentrate feed mixture with Moringa oleifera leaves silage and Chlorella vulgaris microalgae mixture in diets of damascus goats: lactation performance, nutrient utilization, and ruminal fermentation. Animal 2022; 12:1589
https://doi.org/10.3390/ani12121589

2. Matra M, Wanapat M. Phytonutrient pellet supplementation enhanced rumen fermentation efficiency and milk production of lactating Holstein-Friesian crossbred cows. Anim Nutr 2022; 9:119ŌĆō26.
https://doi.org/10.1016/j.aninu.2021.12.002


3. Wanapat M, Viennasay B, Matra M, et al. Supplementation of fruit peel pellet containing phytonutrients to manipulate rumen pH, fermentation efficiency, nutrient digestibility and microbial protein synthesis. J Sci Food Agric 2021; 101:4543ŌĆō50.
https://doi.org/10.1002/jsfa.11096


4. Singh S, Hundal JS, Patra AK, Sethi RS, Sharma A. A composite polyphenol-rich extract improved growth performance, ruminal fermentation and immunity, while decreasing methanogenesis and excretion of nitrogen and phosphorus in growing buffaloes. Environ Sci Pollut Res 2022; 29:24757ŌĆō73.
https://doi.org/10.1007/s11356-021-17674-1

5. Vasta V, Luciano G. The effects of dietary consumption of plants secondary compounds on small ruminantsŌĆÖ products quality. Small Rumin Res 2011; 101:150ŌĆō9.
https://doi.org/10.1016/j.smallrumres.2011.09.035

6. Eisenman SW. The botany of Mitragyna speciosa (Korth.) Havil. and related species. Raffa RB, editorKratom and other Mitragynines: The chemistry and pharmacology of opioids from a non-opium source. Boca Raton, FL, USA: CRC Press; 2015. p. 57ŌĆō76.
7. Singh D, Chear NJY, Narayanan S, et al. Patterns and reasons for kratom (Mitragyna speciosa) use among current and former opioid poly-drug users. J Ethnopharmacol 2020; 249:112462
https://doi.org/10.1016/j.jep.2019.112462


8. Raffa RB. Kratom and other mitragynines: the chemistry and pharmacology of opioids from a non-opium source. Boca Raton, FL, USA: CRC Press; 2015.
9. Hassan Z, Muzaimi M, Navaratnam V, et al. From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev 2013; 37:138ŌĆō51.
https://doi.org/10.1016/j.neubiorev.2012.11.012


10. Phesatcha K, Phesatcha B, Wanapat M, Cherdthong A. Mitragyna speciosa korth leaves supplementation on feed utilization, rumen fermentation efficiency, microbial population, and methane production in vitro. Fermentation 2022; 8:8
https://doi.org/10.3390/fermentation8010008

11. Chanjula P, Wungsintaweekul J, Chiarawipa R, et al. Effect of feed supplement containing dried kratom leaves on apparent digestibility, rumen fermentation, serum antioxidants, hematology, and nitrogen balance in goats. Fermentation 2022; 8:131
https://doi.org/10.3390/fermentation8030131

12. Kim TB, Lee JS, Cho SY, Lee HG. In vitro and in vivo studies of rumen-protected microencapsulated supplement comprising linseed oil, vitamin e, rosemary extract, and hydrogenated palm oil on rumen fermentation, physiological profile, milk yield, and milk composition in dairy cows. Animal 2020; 10:1631
https://doi.org/10.3390/ani10091631

13. Vakarelova M, Zanoni F, Lardo P, et al. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem 2017; 221:289ŌĆō95.
https://doi.org/10.1016/j.foodchem.2016.10.085


14. Flores FP, Singh RK, Kerr WL, Pegg RB, Kong F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem 2014; 153:272ŌĆō8.
https://doi.org/10.1016/j.foodchem.2013.12.063


15. Nouri A. Chitosan nano-encapsulation improves the effects of mint, thyme, and cinnamon essential oils in broiler chickens. Br Poult Sci 2019; 60:530ŌĆō8.
https://doi.org/10.1080/00071668.2019.1622078


16. Kurek MA, Pratap-Singh A. Plant-based (hemp, pea and rice) proteinŌĆōmaltodextrin combinations as wall material for spray-drying microencapsulation of hempseed (Cannabis sativa) oil. Foods 2020; 9:1707
https://doi.org/10.3390/foods9111707



17. Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm 2002; 249:165ŌĆō14.
https://doi.org/10.1016/S0378-5173(02)00487-8


18. AOAC. Official methods of analysis. 19th edGaithersburg, MD, USA: Association of Official Analytical Chemists; 2012.
19. Van Soest PV, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 1991; 74:3583ŌĆō97.
https://doi.org/10.3168/jds.S0022-0302(91)78551-2


20. Al-Duais M, M├╝ller L, B├Čhm V, Jetschke G. Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: use of different assays. Eur Food Res Technol 2009; 228:813ŌĆō21.
https://doi.org/10.1007/s00217-008-0994-8

21. Top├¦u G, Ay M, Bilici A, Sar─▒k├╝rkc├╝ C, ├¢zt├╝rk M, Ulubelen A. A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chem 2007; 103:816ŌĆō22.
https://doi.org/10.1016/j.foodchem.2006.09.028

22. Gali L, Bedjou F. Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. S Afr J Bot 2019; 120:163ŌĆō9.
https://doi.org/10.1016/j.sajb.2018.04.011

23. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26:1231ŌĆō7.
https://doi.org/10.1016/S0891-5849(98)00315-3


24. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ŌĆ£antioxidant powerŌĆØ: the FRAP assay. Anal Biochem 1996; 239:70ŌĆō6.
https://doi.org/10.1006/abio.1996.0292


25. Phupaboon S, Matra M, Prommachart R, Totakul P, Supapong C, Wanapat M. Extraction, characterization, and chitosan microencapsulation of bioactive compounds from Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K. Antioxidants 2022; 11:2103
https://doi.org/10.3390/antiox11112103



26. National Research Council (NRC). Nutrient requirements of dairy cattle. 7th edNational Research Council; Washington, DC, USA: The National Academies Press; 2001.
27. Matra M, Totakul P, Wanapat M. Utilization of dragon fruit waste by-products and non-protein nitrogen source: Effects on in vitro rumen fermentation, nutrients degradability and methane production. Livest Sci 2021; 243:104386
https://doi.org/10.1016/j.livsci.2020.104386

28. ├śrskov ER, McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci 1979; 92:499ŌĆō503.
https://doi.org/10.1017/S0021859600063048

29. Samuel M, Ceballos-Baumann AO, Blin J, et al. Evidence for lateral premotor and parietal overactivity in ParkinsonŌĆÖs disease during sequential and bimanual movements. A PET study. Brain 1997; 120:963ŌĆō76.
https://doi.org/10.1093/brain/120.6.963


30. Koike S, Kobayashi Y. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol Lett 2001; 204:361ŌĆō6.
https://doi.org/10.1111/j.1574-6968.2001.tb10911.x


31. Ouwerkerk D, Klieve AV, Forster RJ. Enumeration of Megasphaera elsdenii in rumen contents by real-time Taq nuclease assay. J Appl Microbiol 2002; 92:753ŌĆō8.
https://doi.org/10.1046/j.1365-2672.2002.01580.x


32. Fernando SC, Purvis HT, Najar FZ, et al. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl Environ Microbiol 2010; 76:7482ŌĆō90.
https://doi.org/10.1128/AEM.00388-10



33. Yu Y, Lee C, Kim J, Hwang S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 2005; 89:670ŌĆō9.
https://doi.org/10.1002/bit.20347


34. Statistical Analysis System. UserŌĆÖs guide: Statistic. Cary, NC, USA: SAS Inst. Inc; 2013.
35. Pal K, Patra AK, Sahoo A. Evaluation of feeds from tropical origin for in vitro methane production potential and rumen fermentation in vitro. Span J Agric Res 2015; 13:e0608
https://doi.org/10.5424/sjar/2015133-7467

36. Zhan J, Liu M, Su X, Zhan K, Zhang C, Zhao G. Effects of alfalfa flavonoids on the production performance, immune system, and ruminal fermentation of dairy cows. Asian-Australas J Anim Sci 2017; 30:1416ŌĆō24.
https://doi.org/10.5713/ajas.16.0579



37. Sommai S, Cherdthong A, Suntara C, So S, Wanapat M, Polyorach S. In vitro fermentation characteristics and methane mitigation responded to flavonoid extract levels from Alternanthera sissoo and dietary ratios. Fermentation 2021; 7:109
https://doi.org/10.3390/fermentation7030109

38. Wanapat M. Manipulation of cassava cultivation and utilization to improve protein to energy biomass for livestock feeding in the tropics. Asian-Australas J Anim Sci 2003; 16:463ŌĆō72.
https://doi.org/10.5713/ajas.2003.463

39. Viennasay B, Totakul P, Matra M, Phesatcha B, Wanapat M. Influence of bamboo grass (Tiliacora triandra, Diels) pellet supplementation on in vitro fermentation and methane mitigation. J Sci Food Agric 2022; 102:4927ŌĆō32.
https://doi.org/10.1002/jsfa.11858


40. Ahmed E, Fukuma N, Hanada M, Nishida T. The efficacy of plant-based bioactives supplementation to different proportion of concentrate diets on methane production and rumen fermentation characteristics in vitro. Animals 2021; 11:1029
https://doi.org/10.3390/ani11041029



41. Patra AK, Saxena J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev 2009; 22:204ŌĆō19.
https://doi.org/10.1017/S0954422409990163


42. Newbold CJ, L├│pez S, Nelson N, Ouda JO, Wallace RJ, Moss AR. Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro. Br J Nutr 2005; 94:27ŌĆō35.
https://doi.org/10.1079/BJN20051445


43. Totakul P, Viennasay B, Sommai S, Matra M, Infascelli F, Wanapat M. Chaya (Cnidoscolus aconitifolius, Mill. Johnston) pellet supplementation improved rumen fermentation, milk yield and milk composition of lactating dairy cows. Livest Sci 2022; 262:104974
https://doi.org/10.1016/j.livsci.2022.104974

44. Bodas R, Prieto N, Garc├Ła-Gonz├Īlez R, Andr├®s S, Gir├Īldez FJ, L├│pez S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim Feed Sci Technol 2012; 176:78ŌĆō93.
https://doi.org/10.1016/j.anifeedsci.2012.07.010

45. Naumann HD, Tedeschi LO, Zeller WE, Huntley NF. The role of condensed tannins in ruminant animal production: advances, limitations and future directions. Rev Bras Zootec 2017; 46:929ŌĆō49.
https://doi.org/10.1590/S1806-92902017001200009

46. Huang H, Szumacher-Strabel M, Patra AK, et al. Chemical and phytochemical composition, in vitro ruminal fermentation, methane production, and nutrient degradability of fresh and ensiled Paulownia hybrid leaves. Anim Feed Sci Technol 2021; 279:115038
https://doi.org/10.1016/j.anifeedsci.2021.115038

47. Ultee A, Kets EPW, Smid EJ. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 1999; 65:4606ŌĆō10.
https://doi.org/10.1128/AEM.65.10.4606-4610.1999



48. Rira M, Morgavi DP, Archim├©de H, et al. Potential of tannin-rich plants for modulating ruminal microbes and ruminal fermentation in sheep. J Anim Sci 2015; 93:334ŌĆō47.
https://doi.org/10.2527/jas.2014-7961


49. Berchez M, Urcan AC, Corcionivoschi N, Criste A. In vitro effects of phenolic acids and IgY immunoglobulins on aspects of rumen fermentation. Rom Biotechnol Lett 2019; 24:513ŌĆō21.
https://doi.org/10.25083/rbl/24.3/513.521

50. Manasri N, Wanapat M, Navanukraw C. Improving rumen fermentation and feed digestibility in cattle by mangosteen peel and garlic pellet supplementation. Livest Sci 2012; 148:291ŌĆō5.
https://doi.org/10.1016/j.livsci.2012.06.009







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





