5. A consumer survey on plant alternatives to animal meat [Internet]. Washington, DC, USA: International Food Information Council; c2020. [cited 2022 Oct 2]. Available from:
www.foodinsight.org
8. Investigation based on plan-based food market in US. Naju, Korea: KATI; 2020.
9. FDA releases list of priority guidance topics for foods program. Silver Spring MD, USA: FDA; 2022.
11. FDA GRASS Notice (GRN) No 737 for Soy Leghemoglobin for protein preparation derived from pich/a pastoris. Silver Spring MD, USA: FDA; c2018. [cited 2022 Nov 16]
19. Center for Agricultured & Food Systems. What’s the beef? debates over cell-cultured meat. c2022. [cited 2022 Dec 20]South Royalton, VT, USA: Vermont Law and Graduate School; 2022.
22. Kyle G. The U.S. plant-based retail market grew at 27 percent over last year, almost two times greater than total retail food sales. Washington, DC, USA: Good Food Institute; 2020.
24. FDA. Formal agreement between FDA and USDA regarding oversight of human food produced using animal cell technology derived from cell lines of USDA-amenable Species. Silver Spring MD, USA: Domestic Interagency Agreements on Food; 2019.
28. Andrieu E. REPORT on the proposal for a regulation of the European Parliament and of the Council amending Regulations (EU) 1308/2013 establishing a common organisation of the markets in agricultural products, (EU) 1151/2012 on quality schemes for agricultural products and foodstuffs, (EU) 251/2014 on the definition, description, presentation, labelling and the protection of geographical indications of aromatised wine products, (EU) 228/2013 laying down specific measures for agriculture in the outermost regions of the Union and (EU) 229/2013 laying down specific measures for agriculture in favour of the smaller Aegean islands. E. Parliament. 2018.
29. European Parliament and Council. Regulation 2015/22 83/EC, On novel foods, amending Regulation (EU) 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Brussel, Belgium: European Parliament; 2015.
30. Processed food market status report - meat processed products. Naju, Korea: Korea Agro-Fisheries & Food Trade Corporation; 2020.
39. KR patent 10-2021-0097716. Method for Extracting Leghemoglobin from Soybean Root and Using the same into Food. Daejeon, Korea: Korean Intellectual Property Office; 2020.
40. KR patent 10-2020-0070921. Artificial meat food composition using vegetable protein, artificial meat and manufacturing method thereof mushroom concentrates and bovine satellite cell culture media. Daejeon, Korea: Korean Intellectual Property Office; 2019.
41. KR patent No 10-2020-0090170. A method for making sausage analogue using mixed bean protein concentrate. Daejeon, Korea: Korean Intellectual Property Office; 2020.
42. Ahmad MI, Farooq S, Alhamoud Y, Li C, Zhang H. A review on mycoprotein: History, nutritional composition, production methods, and health benefits. Trends Food Sci Technol 2022; 121:14–29.
https://doi.org/10.1016/j.tifs.2022.01.027

43. Derbyshire EJ, Delange J. Fungal protein–what is it and what is the health evidence? A systematic review focusing on mycoprotein. Front Sustain Food Syst 2021; 5:581682
https://doi.org/10.3389/fsufs.2021.581682

44. Maurya NK, Kushwaha R. Chapter-7 Novel protein foods: alternative sources of protein for human consumption. Research trends in food technology and nutrition. 4:New Delhi, India: Akinik Puplication; 2019. p. 129–42.
45. Salgado CL, Muñoz R, Blanco A, Lienqueo ME. Valorization and upgrading of the nutritional value of seaweed and seaweed waste using the marine fungi Paradendryphiella salina to produce mycoprotein. Algal Res 2021; 53:102135
https://doi.org/10.1016/j.algal.2020.102135

47. Landeta-Salgado C, Cicatiello P, Lienqueo ME. Mycoprotein and hydrophobin like protein produced from marine fungi Paradendryphiella salina in submerged fermentation with green seaweed Ulva spp. Algal Res 2021; 56:102314
https://doi.org/10.1016/j.algal.2021.102314

49. Stoffel F, de Oliveira Santana W, Gregolon JGN, Kist TBL, Fontana RC, Camassola M. Production of edible mycoprotein using agroindustrial wastes: Influence on nutritional, chemical and biological properties. Innov Food Sci Emerg Technol 2019; 58:102227
https://doi.org/10.1016/j.ifset.2019.102227

52. Jeong JY, Jo C. The application of meat alternatives and ingredients for meat and processed meat industry. Animal Food Science and Industry 2018; 7:2–11.
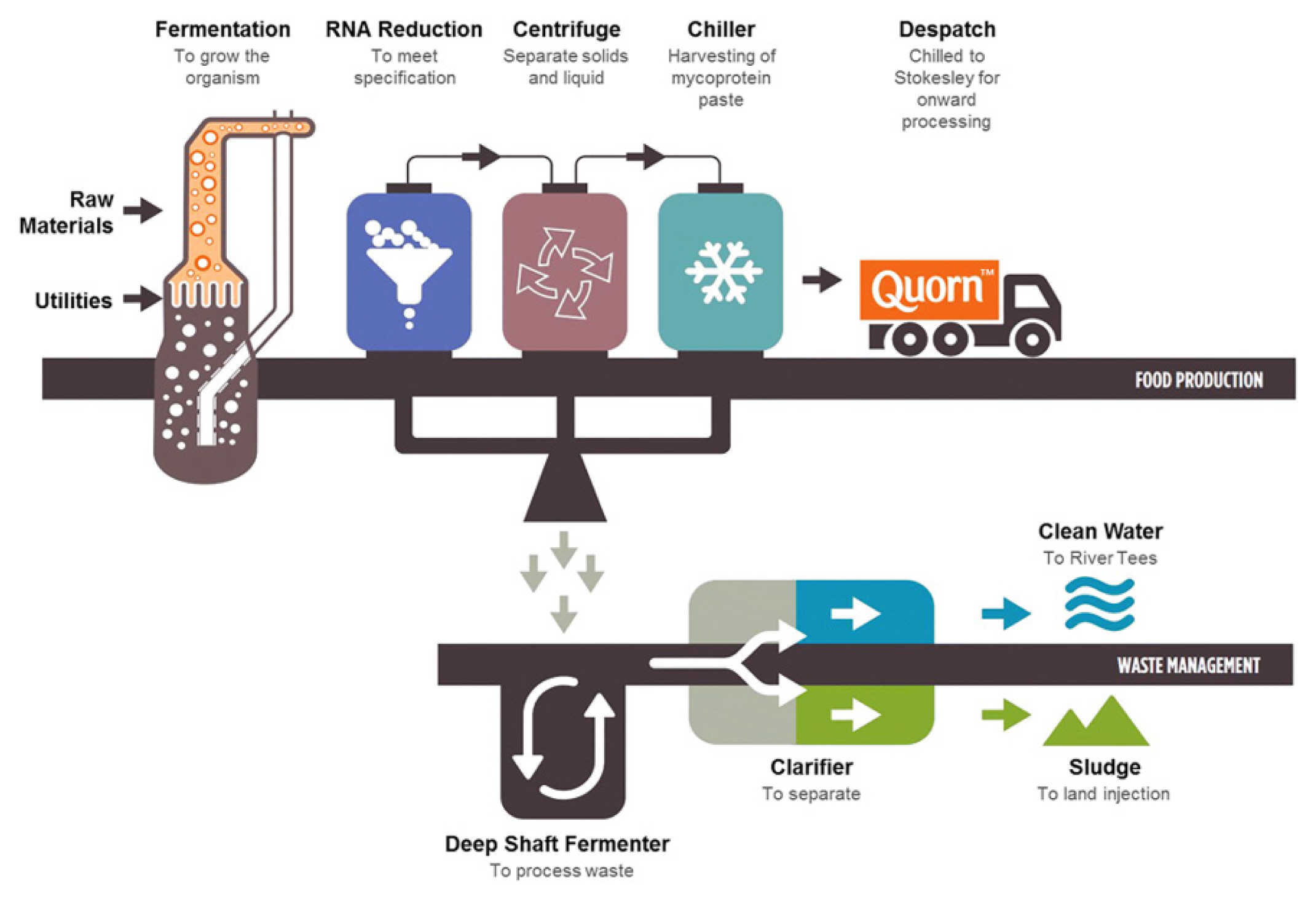
53. Lonchamp J, Clegg PS, Euston SR. Foaming, emulsifying and rheological properties of extracts from a co-product of the Quorn fermentation process. Euro Food Res Technol 2019; 245:1825–39.
https://doi.org/10.1007/s00217-019-03287-z

54. Finnigan TJA. Mycoprotein: origins, production and properties. Handbook of food proteins. Philadelphia, PA, USA: Woodhead Publishing; 2011. p. 335–52.

55. Lonchamp J, Clegg PS, Euston SR. Foaming, emulsifying and rheological properties of extracts from a co-product of the Quorn fermentation process. Eur Food Res Technol 2019; 245:1825–39.
https://doi.org/10.1007/s00217-019-03287-z

56. Park YK, Nicaud JM, Ledesma-Amaro R. The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol 2018; 36:304–17.
https://doi.org/10.1016/j.tibtech.2017.10.013


57. Lee JJ, Chen L, Cao B, Chen WN. Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture. Appl Microbiol Biotechnol 2016; 100:869–77.
https://doi.org/10.1007/s00253-015-7102-3


58. Belluco S, Losasso C, Maggioletti M, Alonzi CC, Paoletti MG, Ricci A. Edible insects in a food safety and nutritional perspective: a critical review. Compr Rev Food Sci Food Saf 2013; 12:296–313.
https://doi.org/10.1111/1541-4337.12014

59. Xia Z, Wu S, Pan S, Kim JM. Nutritional evaluation of protein from Clanis bilineata (Lepidoptera), an edible insect. J Sci Food Agric 2012; 92:1479–82.
https://doi.org/10.1002/jsfa.4730


61. Xiaoming C, Ying F, Hong Z, Zhiyong C. Review of the nutritive value of edible insects. In : Proceedings of a workshop on Asia-Pacific resources and their potential for development; Chiang Mai, Thailand. 19–21 February, 2008; Forest insects as food: humans bite back. Rome, Italy: FAO; 2010. p. 85
62. Mishyna M, Martinez JJI, Chen J, Benjamin O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res Int 2019; 116:697–706.
https://doi.org/10.1016/j.foodres.2018.08.098


63. Purschke B, Meinlschmidt P, Horn C, Rieder O, Jäger H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur Food Res Technol 2018; 244:999–1013.
https://doi.org/10.1007/s00217-017-3017-9

64. Lee HS, Ryu HJ, Song HJ, Lee SO. Enzymatic preparation and antioxidant activities of protein hydrolysates from Protaetia brevitarsis larvae. J Korean Soc Food Sci Nutr 2017; 46:1164–70.
https://doi.org/10.3746/jkfn.2017.46.10.1164

65. De Marchi L, Mainente F, Leonardi M, et al. Allergenicity assessment of the edible cricket Acheta domesticus in terms of thermal and gastrointestinal processing and IgE cross-reactivity with shrimp. Food Chem 2021; 359:129878
https://doi.org/10.1016/j.foodchem.2021.129878


67. Poma G, Cuykx M, Amato E, Calaprice C, Focant JF, Covaci A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem Toxicol 2017; 100:70–9.
https://doi.org/10.1016/j.fct.2016.12.006


72. Kim YJ, Kim TK, Cha JY, et al. Consumer awareness survey analysis of alternative protein: Cultured meat and edible insect. Food Life 2022; 2022:89–95.
https://doi.org/10.5851/fl.2022.e11

76. KR patent 10-2016-0150130. Serum-free medium for animal cell culture. Daejeon, Korea: Korean Intellectual Property Office; 2018.
77. KR patent 10-2017-0120431. Manufacturing method of patty using mushroom concentrates and bovine satellite cell culture media. Daejeon, Korea: Korean Intellectual Property Office; 2018.
84. Hoek AC, Luning PA, Weijzen P, Engels W, Kok FJ, De Graaf C. Replacement of meat by meat substitutes. A survey on person-and product-related factors in consumer acceptance. Appetite 2011; 56:662–73.
https://doi.org/10.1016/j.appet.2011.02.001


86. Martínez-Villaluenga C, Gulewicz P, Frias J, Gulewicz K, Vidal-Valverde C. Assessment of protein fractions of three cultivars of Pisum sativum L.: Effect of germination. Eur Food Res Technol 2008; 226:1465–78.
https://doi.org/10.1007/s00217-007-0678-9

88. Franca PAP, Duque-Estrada P, e Sá BF, van der Goot AJ, Pierucci APTR. Meat substitutes-past, present, and future of products available in Brazil: changes in the nutritional profile. Future Foods 2022; 5:100133
https://doi.org/10.1016/j.fufo.2022.100133













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print







