 |
 |
| Anim Biosci > Volume 36(8); 2023 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Yan C is an employee of Yanbian Hongchao Smart Animal Husbandry Co., Ltd.
FUNDING
This work was carried out with the support of Major Science and Technology Special Project for the Industrialization and Development of Beef Cattle in Jilin Province, China (Grant number YDZJ202203CGZH043) and Yanbian University Research Program (Grant number ydxq202207) and Scientific Research Project of Education Department of Jilin Province (Grant number JJKH20230629KJ). This research also was supported by the Research Fund of Engineering Research Center of North-East Cold Region Beef Cattle Science and Technology Innovation, Ministry of Education and the ŌĆ£111ŌĆØ Project (D20034), China.
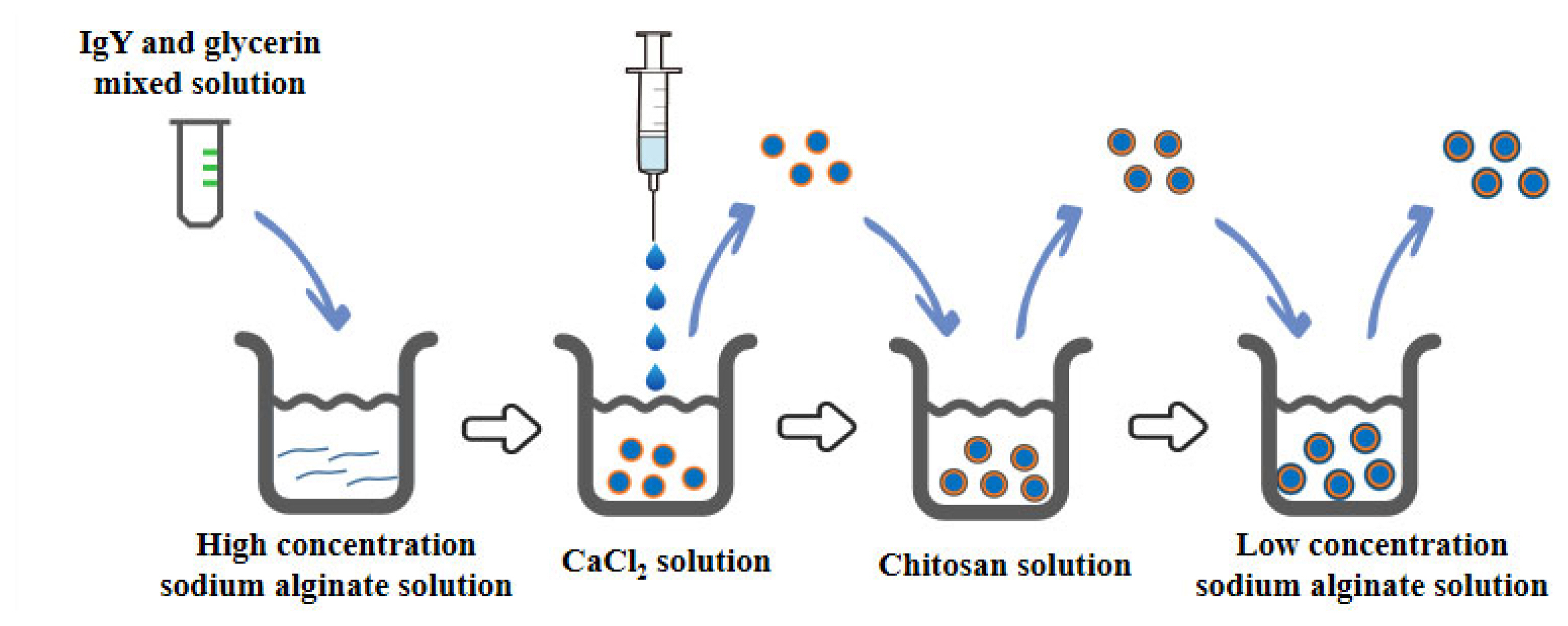
Figure┬Ā1
Table┬Ā1
| Items | Composition (%) |
|---|---|
| Ingredient | |
| ŌĆāCorn | 64.29 |
| ŌĆāSoybean meal | 27.55 |
| ŌĆāCorn protein powder | 3.1 |
| ŌĆāSalt | 0.26 |
| ŌĆāCalcium hydrogen phosphate | 1.49 |
| ŌĆāMineral meal | 0.11 |
| ŌĆāBran | 2.2 |
| ŌĆāVitamin and mineral premixes1) | 1 |
| ŌĆāTotal | 100 |
| Calculated chemical composition2) | |
| ŌĆāMetabolic energy (MJ/kg) | 2.83 |
| ŌĆāCrude protein | 20.95 |
| ŌĆāLysine | 0.92 |
| ŌĆāMethionine | 0.32 |
| ŌĆāCalcium | 0.59 |
| ŌĆāTotal phosphorus | 0.73 |
| ŌĆāNon-phytate phosphorus | 0.48 |
1) Provided the following quantities per kg of complete diet: vitamin A 10,999 IU; vitamin D3 3,000 IU; vitamin E 15 IU; vitamin K 319 mg; vitamin B 110 mg; vitamin B2 30 mg; vitamin B6 20 mg; vitamin B12 0.2 mg; nicotinic acid 599 mg; pantothenic acid 181 mg; folic acid 10.1 mg; biotin 0.79 mg; Choline 6.99 mg; Cu 0.2 g; Fe 1.2 g; Mn 1.9 g; Zn 1.8 g; I 10 mg; Se 5.99 mg.
Table┬Ā2
| Item | Dietary treatment2) | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NC | CH | NM | LM | HM | |||
| Initial body weight (g) | 45.6 | 44.0 | 45.7 | 44.6 | 44.2 | 0.488 | 0.735 |
| Final body weight (g) | 1,079.3b | 1,220.9a | 1,249.9a | 1,215.1a | 1,287.1a | 15.057 | <0.001 |
| Day 1 to 14 | |||||||
| ŌĆāAverage daily gain (g/d) | 18.98 | 19.22 | 20.06 | 19.42 | 20.52 | 0.380 | 0.708 |
| ŌĆāAverage daily feed intake (g/d) | 35.29 | 35.03 | 36.44 | 35.05 | 34.42 | 0.420 | 0.665 |
| ŌĆāFeed conversion ratio | 1.89 | 1.84 | 1.86 | 1.82 | 1.72 | 0.039 | 0.724 |
| Day 14 to 28 | |||||||
| ŌĆāAverage daily gain (g/d) | 54.86b | 64.84a | 65.95a | 64.19a | 68.26a | 1.133 | 0.001 |
| ŌĆāAverage daily feed intake (g/d) | 107.11 | 108.83 | 106.44 | 105.33 | 104.46 | 0.530 | 0.082 |
| ŌĆāFeed conversion ratio | 2.00a | 1.70b | 1.62b | 1.65b | 1.54b | 0.036 | <0.001 |
| Day 1 to 28 | |||||||
| ŌĆāAverage daily gain (g/d) | 36.92b | 42.03a | 43.00a | 41.80a | 44.39a | 0.540 | <0.001 |
| ŌĆāAverage daily feed intake (g/d) | 71.20 | 71.93 | 71.44 | 70.19 | 69.44 | 0.362 | 0.185 |
| ŌĆāFeed conversion ratio | 1.94a | 1.72b | 1.66bc | 1.68bc | 1.57c | 0.026 | <0.001 |
Table┬Ā3
| Item | Dietary treatment2) | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NC | CH | NM | LM | HM | |||
| Relative weight (g/kg BW) | |||||||
| ŌĆāDuodenum | 8.91 | 8.58 | 8.95 | 8.62 | 8.55 | 0.114 | 0.699 |
| ŌĆāJejunum | 15.92 | 15.65 | 15.76 | 15.11 | 15.13 | 0.206 | 0.640 |
| ŌĆāIleum | 2.22 | 2.29 | 2.38 | 2.34 | 2.21 | 0.050 | 0.79 |
| ŌĆāSpleen | 1.22c | 1.31ab | 1.25bc | 1.32a | 1.36a | 0.013 | 0.002 |
| ŌĆāThymus | 2.81b | 3.08ab | 2.95ab | 3.16a | 3.21a | 0.047 | 0.039 |
| ŌĆāBursa of Fabricius | 2.00 | 2.07 | 2.03 | 2.13 | 2.15 | 0.025 | 0.225 |
| Relative length (cm/kg BW) | |||||||
| ŌĆāDuodenum | 32.8 | 32.01 | 33.17 | 31.62 | 32.55 | 0.333 | 0.616 |
| ŌĆāJejunum | 62.23 | 60.30 | 59.88 | 61.71 | 63.31 | 0.654 | 0.461 |
| ŌĆāIleum | 11.09 | 11.15 | 10.59 | 10.64 | 10.84 | 0.165 | 0.768 |
IgY, immunoglobulin Y; SA, alginate; CS, chitosan; SEM, standard error of the mean; BW, body weight.
Table┬Ā4
| Item | Dietary treatment2) | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NC | CH | NM | LM | HM | |||
| Duodenum | |||||||
| ŌĆāVH (╬╝m) | 1,365.0b | 1,449.6a | 1,377.9b | 1,460.8a | 1,491.8a | 10.396 | <0.001 |
| ŌĆāCD (╬╝m) | 175.4 | 170.9 | 167.4 | 169.1 | 158.6 | 2.286 | 0.211 |
| ŌĆāVH:CD | 7.83c | 8.53bc | 8.28bc | 8.76ab | 9.45a | 0.133 | 0.001 |
| Jejunum | |||||||
| ŌĆāVH (╬╝m) | 943.2c | 971.3bc | 951.5c | 1,003.3ab | 1,027.8a | 7.910 | 0.001 |
| ŌĆāCD (╬╝m) | 152.5 | 150.2 | 145.3 | 133.9 | 132.7 | 3.219 | 0.163 |
| ŌĆāVH:CD | 6.29c | 6.63bc | 6.74bc | 7.58ab | 7.92a | 0.172 | 0.008 |
| Ileum | |||||||
| ŌĆāVH (╬╝m) | 974.2b | 993.4b | 1,004.4b | 1,073.0a | 1,110.4a | 12.193 | <0.001 |
| ŌĆāCD (╬╝m) | 162.2 | 160.0 | 157.1 | 152.6 | 151.2 | 2.142 | 0.439 |
| ŌĆāVH:CD | 6.04c | 6.29c | 6.44bc | 7.10ab | 7.42a | 0.134 | 0.002 |
IgY, immunoglobulin Y; SA, alginate; CS, chitosan; SEM, standard error of the mean; VH, villus height; CD, crypt depth.
Table┬Ā5
| Item | Dietary treatment2) | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NC | CH | NM | LM | HM | |||
| TP (g/L) | 24.24 | 24.59 | 25.32 | 24.09 | 26.43 | 0.374 | 0.264 |
| ALB (g/L) | 12.84c | 14.02ab | 13.16bc | 13.80a | 14.40a | 0.154 | 0.005 |
| TC (mmol/L) | 2.77 | 2.92 | 2.81 | 2.92 | 2.99 | 0.034 | 0.246 |
| TG (mmol/L) | 0.36 | 0.38 | 0.38 | 0.41 | 0.37 | 0.006 | 0.253 |
| GLU (mmol/L) | 10.37 | 10.58 | 10.87 | 10.87 | 10.69 | 0.109 | 0.568 |
| ALP (U/L) | 2,509.88 | 2,564.49 | 2,432.75 | 2,556.76 | 2,633.02 | 34.069 | 0.449 |
IgY, immunoglobulin Y; SA, alginate; CS, chitosan; SEM, standard error of the mean; TP, total protein; ALB, albumin; TC, total cholesterol; TG, triglyceride; GLU, gLucose; ALP, alkaline phosphatase.
Table┬Ā6
| Item | Dietary treatment2) | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NC | CH | NM | LM | HM | |||
| IgA (g/L) | 2.34c | 2.65bc | 2.55c | 2.92ab | 3.09a | 0.061 | <0.001 |
| IgG (g/L) | 3.69c | 4.43b | 4.47b | 4.62ab | 4.92a | 0.086 | <0.001 |
| IgM (g/L) | 2.17b | 2.46ab | 2.47a | 2.62a | 2.77a | 0.056 | 0.009 |
IgY, immunoglobulin Y; SA, alginate; CS, chitosan; SEM, standard error of the mean; IgA, immunoglobulinA, IgG, immunoglobulinG; IgM, immunoglobulin M.
Table┬Ā7
| Item | Dietary treatment2) | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NC | CH | NM | LM | HM | |||
| MDA (nmol/mL) | 3.23a | 2.68b | 2.74b | 2.59bc | 2.30c | 0.070 | <0.001 |
| SOD (U/mL) | 47.62c | 58.56b | 54.6b | 56.16b | 64.33a | 1.043 | <0.001 |
| T-AOC (U/mL) | 9.36b | 9.49b | 9.73b | 9.95b | 11.35a | 0.167 | <0.001 |
| GSH-Px (U/mL) | 702.08c | 748.96b | 755.59b | 776.23ab | 809.67a | 8.515 | 0.001 |
IgY, immunoglobulin Y; SA, alginate; CS, chitosan; SEM, standard error of the mean; MDA, Malondialdehyde; SOD, Superoxide dismutase; T-AOC, total antioxidant capacity; GSH-Px, glutathione peroxidase.
Table┬Ā8
| Item (lg cfu/g) | Dietary treatment2) | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NC | CH | NM | LM | HM | |||
| Escherichia coli | 8.55a | 7.64b | 7.78b | 7.52b | 7.34b | 0.104 | 0.001 |
| Salmonella Gallinarum | 8.41a | 7.31b | 7.46b | 7.56b | 7.69b | 0.105 | 0.006 |
| Lactic acid bacteria | 8.05c | 8.35bc | 8.16c | 8.56b | 9.18a | 0.085 | <0.001 |
| Bifidobacterium | 9.50b | 9.60b | 9.57b | 9.48b | 10.01a | 0.061 | 0.034 |






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





