 |
 |
| Anim Biosci > Volume 34(4); 2021 > Article |
|
Abstract
Objective
Methods
Results
ACKNOWLEDGMENTS
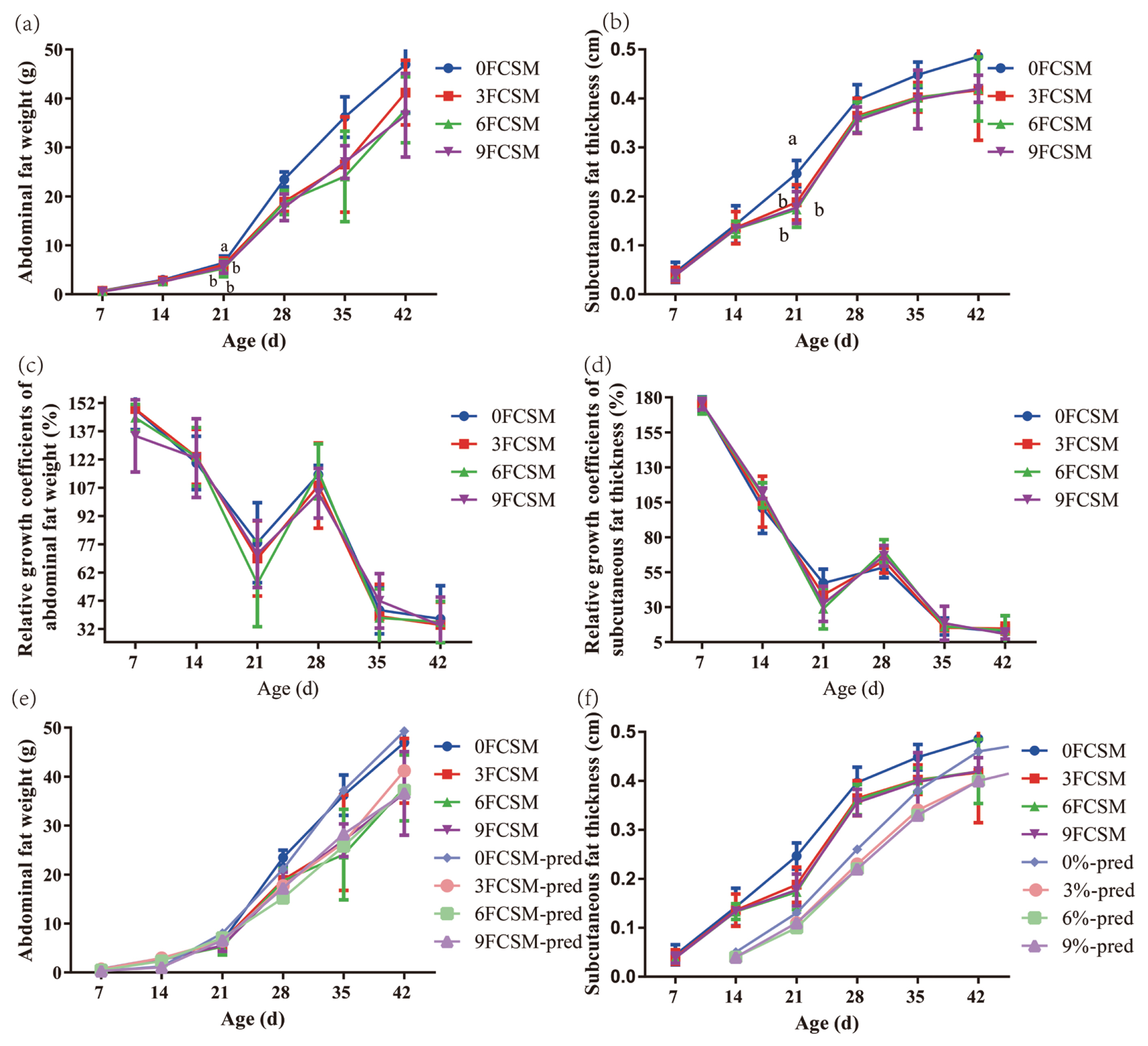
Figure┬Ā1
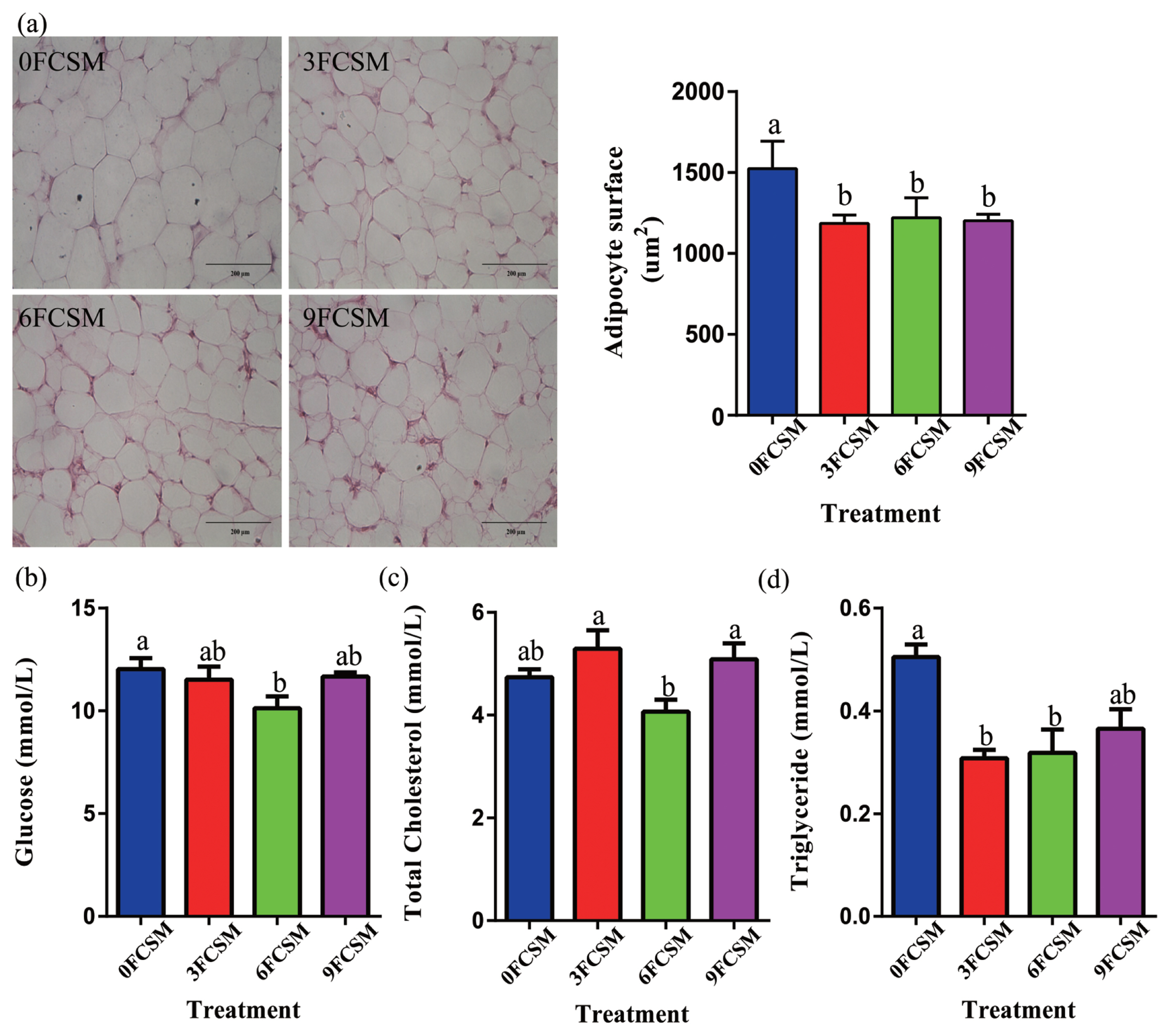
Figure┬Ā2
Table┬Ā1
| Items (g/kg) | 1 to 3 wk1) | 4 to 6 wk1) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0FCSM | 3FCSM | 6FCSM | 9FCSM | 0FCSM | 3FCSM | 6FCSM | 9FCSM | |
| Ingredient | ||||||||
| ŌĆāYellow corn | 545.0 | 545.0 | 543.0 | 543.0 | 582.0 | 582.0 | 581.5 | 580.0 |
| ŌĆāSoybean meal | 335.0 | 304.0 | 274.0 | 243.0 | 288.0 | 257.0 | 226.5 | 196.0 |
| ŌĆāFermented cottonseed meal | 0.0 | 30.0 | 60.0 | 90.0 | 0.0 | 30.0 | 60.0 | 90.0 |
| ŌĆāSunflower oil | 30.0 | 31.0 | 33.0 | 34.0 | 40.0 | 41.0 | 42.0 | 44.0 |
| ŌĆāCottonseed protein | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| ŌĆāPremix2) | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Nutrient content3) | ||||||||
| ŌĆāMetabolizable energy (Mcal/kg) | 2.95 | 2.95 | 2.95 | 2.95 | 3.06 | 3.05 | 3.05 | 3.05 |
| ŌĆāCrude protein | 212.3 | 212.1 | 212.3 | 212.1 | 195.2 | 195.1 | 195.2 | 195.1 |
| ŌĆāEther extract | 55.7 | 55.4 | 56.0 | 55.8 | 65.9 | 65.8 | 66.1 | 66.0 |
| ŌĆāCrude ash | 72.2 | 72.3 | 72.4 | 72.5 | 71.2 | 71.3 | 71.3 | 71.4 |
| ŌĆāCrude fiber | 31.1 | 31.7 | 32.3 | 32.9 | 29.1 | 29.7 | 30.3 | 30.9 |
| ŌĆāCalcium | 10.4 | 10.3 | 10.3 | 10.3 | 9.6 | 9.6 | 9.6 | 9.5 |
| ŌĆāTotal phosphorus | 6.9 | 7.0 | 7.1 | 7.2 | 6.4 | 6.5 | 6.6 | 6.7 |
| ŌĆāAvailable phosphate | 4.5 | 4.5 | 4.5 | 4.6 | 4.1 | 4.1 | 4.1 | 4.2 |
| ŌĆāMethionine | 5.0 | 5.0 | 5.0 | 5.0 | 4.8 | 4.8 | 4.8 | 4.8 |
| ŌĆāMethionine+cysteine | 8.6 | 8.6 | 8.6 | 8.6 | 8.2 | 8.2 | 8.2 | 8.2 |
| ŌĆāThreonine | 7.8 | 7.7 | 7.6 | 7.4 | 7.1 | 7.0 | 6.9 | 6.7 |
| ŌĆāLysine | 11.2 | 10.9 | 10.8 | 10.5 | 10.0 | 9.8 | 9.6 | 9.4 |
| ŌĆāFree gossypol | 16.0 | 17.1 | 18.2 | 19.3 | 16.0 | 17.1 | 18.2 | 19.3 |
1) 0FCSM, 0% fermented cottonseed meal in diet; 3FCSM, 3% fermented cottonseed meal in diet; 6FCSM, 6% fermented cottonseed meal in diet; 9FCSM, 9% fermented cottonseed meal in diet.
2) Premix provided the following per kg of diets: CaCO3 10.67 g (1 to 21 d), 11.34 g (21 to 42 d), CaHPO4 13.07 g (1 to 21 d), 9.8 g (21 to 42 d); NaCl 2.94 g, Lys 0.234 g, Met 1.791 g, retinol 8,800 IU, cholecalciferol 3,000 IU, ɑ-tocopherol 30 mg, menaquinone 1.65 mg, thiamin 2.5 mg, riboflavin 6.6 mg, nicotinic acid 11 mg, adenine 500 mg, pantothenic acid 60 mg, pyridoxine 4.0 mg, biotin 0.2 mg, folic acid 1.0 mg, cyanocobalamin 0.02 mg, ascorbic acid 50 mg, Fe 80.0 mg, Cu 8.0 mg, Zn 60.0 mg, Mn 70.0 mg, I 0.5 mg, Se 0.3 mg.
Table┬Ā2
Table┬Ā3
1) Y is the expected abdominal fat weight or subcutaneous fat thickness at the week of t; A is the maximum abdominal fat weight or subcutaneous fat thickness when age approaches infinity; k is the coefficient of relative growth or maturing index; B is the integration constant which is related to hatching weight or thickness; t is time (age); W is the inflection point weight; Wi is the weight in week i.
Table┬Ā4
| Items | Treatments1) | SEM | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0FCSM | 3FCSM | 6FCSM | 9FCSM | Treatment | Linear | Quadratic | Cubic | ||
| d 1 to 7 | |||||||||
| ŌĆāADFI (g/d) | 17.98 | 20.28 | 16.66 | 17.03 | 1.79 | 0.81 | 0.66 | 0.69 | 0.44 |
| ŌĆāADG (g/d) | 11.98 | 13.03 | 11.35 | 11.01 | 1.44 | 0.39 | 0.06 | 0.06 | 0.49 |
| ŌĆāFCR (g/g) | 1.62 | 1.56 | 1.48 | 1.59 | 0.31 | 0.32 | 0.06 | 0.06 | 0.17 |
| ŌĆāMortality rate (%)2) | 1.70 | 0.89 | 1.70 | 1.70 | 1.39 | 0.98 | - | - | - |
| d 8 to 14 | |||||||||
| ŌĆāADFI (g/d) | 41.36 | 41.48 | 41.61 | 42.16 | 1.30 | 0.85 | 0.97 | 0.65 | 0.46 |
| ŌĆāADG (g/d) | 23.79 | 24.58 | 24.69 | 24.87 | 0.79 | 0.22 | 0.64 | 0.06 | 0.47 |
| ŌĆāFCR (g/g) | 1.74 | 1.68 | 1.68 | 1.69 | 0.07 | 0.34 | 0.12 | 0.07 | 0.87 |
| ŌĆāMortality rate (%) | 0.89 | 0.00 | 0.89 | 0.00 | 1.01 | 0.76 | - | - | - |
| d 15 to 21 | |||||||||
| ŌĆāADFI (g/d) | 64.61 | 60.02 | 54.32 | 53.11 | 6.58 | 0.74 | 0.33 | 0.65 | 0.91 |
| ŌĆāADG (g/d) | 38.02 | 41.05 | 42.09 | 37.82 | 2.24 | 0.87 | 0.49 | 0.76 | 0.76 |
| ŌĆāFCR (g/g) | 1.69a | 1.46ab | 1.29b | 1.41ab | 0.16 | 0.04 | 0.04 | 0.06 | 0.99 |
| ŌĆāMortality rate (%) | 0.00 | 0.93 | 0.00 | 0.00 | - | - | - | - | - |
| d 22 to 28 | |||||||||
| ŌĆāADFI (g/d) | 97.73 | 103.6 | 93.85 | 91.67 | 6.69 | 0.76 | 0.46 | 0.84 | 0.44 |
| ŌĆāADG (g/d) | 45.40 | 55.33 | 50.59 | 47.36 | 3.52 | 0.48 | 0.82 | 0.64 | 0.20 |
| ŌĆāFCR (g/g) | 2.15 | 1.87 | 1.85 | 1.94 | 0.21 | 0.49 | 0.50 | 0.39 | 0.42 |
| ŌĆāMortality rate (%) | 1.00 | 1.00 | 0.00 | 1.00 | 0.37 | 0.88 | - | - | - |
| d 29 to 35 | |||||||||
| ŌĆāADFI (g/d) | 111.15ab | 124.24a | 120.88ab | 99.13b | 7.78 | 0.05 | 0.19 | 0.03 | 0.91 |
| ŌĆāADG (g/d) | 74.54a | 76.43a | 74.50a | 57.72b | 4.38 | 0.03 | 0.05 | 0.10 | 0.55 |
| ŌĆāFCR (g/g) | 1.71 | 1.63 | 1.62 | 1.72 | 0.12 | 0.55 | 0.35 | 0.35 | 0.64 |
| ŌĆāMortality rate (%) | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - | - |
| d 36 to 42 | |||||||||
| ŌĆāADFI (g/d) | 140.78a | 141.97a | 137.13a | 106.99b | 5.75 | 0.01 | 0.01 | 0.06 | 0.38 |
| ŌĆāADG (g/d) | 70.19 | 77.06 | 75.66 | 60.62 | 5.26 | 0.81 | 0.87 | 0.23 | 0.29 |
| ŌĆāFCR (g/g) | 2.01a | 1.85ab | 1.81ab | 1.75b | 0.12 | 0.08 | 0.03 | 0.07 | 0.07 |
| ŌĆāMortality rate (%) | 1.18 | 2.29 | 1.18 | 1.18 | 1.05 | 0.59 | - | - | - |
| d 1 to 42 | |||||||||
| ŌĆāADFI (g/d) | 78.93 | 81.93 | 76.14 | 70.78 | 6.81 | 0.59 | 0.22 | 0.55 | 0.83 |
| ŌĆāADG (g/d) | 43.23 | 46.17 | 42.18 | 39.42 | 4.90 | 0.13 | 0.06 | 0.19 | 0.66 |
| ŌĆāFCR (g/g) | 1.83 | 1.78 | 1.80 | 1.80 | 0.04 | 0.56 | 0.75 | 0.16 | 0.94 |
| Cumulative mortality rate (%) | 4.17 | 4.17 | 3.30 | 3.30 | 2.32 | 0.62 | - | - | - |
| Cost of diet (YUAN/kg) | 2.82 | 2.82 | 2.82 | 2.82 | - | - | - | - | - |
| Cost of diet per kg of broiler meat (YUAN/kg) | 5.14 | 5.00 | 5.09 | 5.06 | - | - | - | - | - |
| Cost of FCSM (YUAN/kg) | 3.2 | - | - | - | - | - | |||
| The meat production affected by FCSM (kg) | 0 | 0.05 | 0.11 | 0.16 | - | - | - | - | - |
1) 0FCSM, 0% fermented cottonseed meal in diet; 3FCSM, 3% fermented cottonseed meal in diet; 6FCSM, 6% fermented cottonseed meal in diet; 9FCSM, 9% fermented cottonseed meal in diet. The values are means of the six replicates.
SEM, standard error of the mean; ADFI, average daily feed intake; ADG, average daily weight gain; FCR, feed conversion ratio (ADFI/ADG).
Table┬Ā5
| Item | Treatments1) | SEM | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0FCSM | 3FCSM | 6FCSM | 9FCSM | Treatment | Linear | Quadratic | Cubic | ||
| d 7 | |||||||||
| ŌĆāCarcass | 87.32 | 89.79 | 88.54 | 88.56 | 1.45 | 0.26 | 0.26 | 0.42 | 0.14 |
| ŌĆāEviscerated | 57.85 | 57.57 | 58.10 | 57.38 | 0.88 | 0.24 | 0.79 | 0.41 | 0.07 |
| ŌĆāSemi-eviscerate | 82.06 | 82.79 | 84.17 | 82.91 | 1.48 | 0.49 | 0.38 | 0.31 | 0.45 |
| ŌĆāLiver | 7.16 | 7.34 | 6.99 | 7.19 | 0.19 | 0.06 | 0.43 | 0.18 | 0.16 |
| ŌĆāBreast muscle | 15.40 | 14.67 | 14.99 | 14.01 | 0.80 | 0.81 | 0.42 | 0.90 | 0.62 |
| ŌĆāThigh muscle | 16.34 | 16.97 | 17.08 | 17.32 | 0.60 | 0.13 | 0.23 | 0.22 | 0.09 |
| d 14 | |||||||||
| ŌĆāCarcass | 87.47b | 87.07b | 89.55a | 87.87b | 0.35 | 0.01 | 0.01 | 0.05 | 0.02 |
| ŌĆāEviscerated | 60.52 | 60.59 | 59.69 | 57.33 | 1.65 | 0.26 | 0.08 | 0.35 | 0.93 |
| ŌĆāSemi-eviscerate | 79.93 | 79.68 | 79.02 | 77.82 | 0.39 | 0.24 | 0.05 | 0.54 | 0.97 |
| ŌĆāLiver | 5.88 | 5.57 | 5.21 | 5.57 | 0.42 | 0.27 | 0.22 | 0.16 | 0.48 |
| ŌĆāBreast muscle | 16.8 | 16.73 | 17.96 | 18.34 | 0.96 | 0.31 | 0.13 | 0.57 | 0.34 |
| ŌĆāThigh muscle | 16.79 | 16.95 | 16.39 | 16.67 | 0.43 | 0.98 | 0.82 | 0.95 | 0.71 |
| d 21 | |||||||||
| ŌĆāCarcass | 88.93a | 88.95a | 88.21a | 86.44b | 0.23 | 0.02 | 0.02 | 0.03 | 0.19 |
| ŌĆāEviscerated | 69.96 | 72.86 | 69.60 | 70.9 | 1.58 | 0.19 | 0.93 | 0.48 | 0.05 |
| ŌĆāSemi-eviscerate | 85.44b | 89.77a | 86.74ab | 87.76ab | 0.95 | 0.02 | 0.47 | 0.13 | 0.05 |
| ŌĆāLiver | 3.81 | 4.18 | 4.12 | 4.21 | 0.30 | 0.49 | 0.23 | 0.43 | 0.53 |
| ŌĆāBreast muscle | 22.27 | 22.34 | 22.46 | 22.73 | 0.47 | 0.99 | 0.74 | 0.92 | 0.99 |
| ŌĆāThigh muscle | 16.83 | 18.21 | 17.17 | 18.37 | 0.93 | 0.54 | 0.37 | 0.92 | 0.25 |
| d 28 | |||||||||
| ŌĆāCarcass | 87.80 | 88.11 | 87.02 | 87.02 | 0.51 | 0.99 | 0.88 | 0.91 | 0.91 |
| ŌĆāEviscerated | 68.94ba | 73.10a | 71.66ab | 68.71b | 0.58 | 0.01 | 0.62 | 0.01 | 0.34 |
| ŌĆāSemi-eviscerate | 81.85b | 87.39a | 87.59a | 83.34ab | 0.92 | 0.05 | 0.53 | 0.01 | 0.90 |
| ŌĆāLiver | 4.50 | 4.31 | 4.51 | 4.56 | 0.15 | 0.73 | 0.62 | 0.48 | 0.46 |
| ŌĆāBreast muscle | 25.83 | 25.94 | 24.07 | 24.59 | 1.21 | 0.67 | 0.34 | 0.87 | 0.45 |
| ŌĆāThigh muscle | 17.15 | 18.28 | 18.30 | 17.82 | 1.13 | 0.78 | 0.62 | 0.38 | 0.88 |
| d 35 | |||||||||
| ŌĆāCarcass | 87.07 | 88.33 | 88.24 | 87.06 | 1.36 | 0.29 | 0.07 | 0.76 | 0.59 |
| ŌĆāEviscerated | 70.70ab | 74.55a | 69.82b | 69.83b | 1.75 | 0.05 | 0.24 | 0.17 | 0.04 |
| ŌĆāSemi-eviscerate | 85.54b | 89.80a | 84.95b | 84.64b | 1.25 | 0.04 | 0.68 | 0.53 | 0.01 |
| ŌĆāLiver | 4.21 | 3.93 | 4.27 | 4.27 | 0.17 | 0.35 | 0.45 | 0.36 | 0.17 |
| ŌĆāBreast muscle | 23.18a | 22.94a | 20.6b | 19.42b | 0.48 | 0.01 | 0.01 | 0.55 | 0.35 |
| ŌĆāThigh muscle | 21.51 | 21.49 | 20.12 | 20.21 | 0.88 | 0.39 | 0.14 | 0.95 | 0.42 |
| d 42 | |||||||||
| ŌĆāCarcass | 87.48b | 90.82a | 90.69a | 88.76ab | 1.34 | 0.05 | 0.18 | 0.01 | 0.55 |
| ŌĆāEviscerated | 75.94 | 76.43 | 74.99 | 73.57 | 1.54 | 0.27 | 0.09 | 0.38 | 0.68 |
| ŌĆāSemi-eviscerate | 90.07 | 90.81 | 90.14 | 89.87 | 0.71 | 0.97 | 0.85 | 0.74 | 0.79 |
| ŌĆāLiver | 3.17 | 3.36 | 3.46 | 3.56 | 0.30 | 0.59 | 0.19 | 0.83 | 0.92 |
| ŌĆāBreast muscle | 23.50b | 25.86a | 22.29b | 22.85b | 0.45 | 0.04 | 0.16 | 0.18 | 0.02 |
| ŌĆāThigh muscle | 21.99 | 22.63 | 21.83 | 22.79 | 0.46 | 0.77 | 0.64 | 0.83 | 0.36 |
Table┬Ā6
| Items | Treatments1) | SEM | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 0FCSM | 3FCSM | 6FCSM | 9FCSM | Treatment | Linear | Quadratic | Cubic | ||
| d 7 | |||||||||
| ŌĆāAbdominal fat | 1.08 | 0.98 | 0.97 | 0.96 | 0.07 | 0.84 | 0.45 | 0.69 | 0.87 |
| ŌĆāSubcutaneous fat thickness | 0.04 | 0.04 | 0.04 | 0.04 | 0.00 | 0.89 | 0.46 | 0.75 | 0.99 |
| d 14 | |||||||||
| ŌĆāAbdominal fat | 1.75 | 1.62 | 1.45 | 1.46 | 0.17 | 0.34 | 0.09 | 0.63 | 0.70 |
| ŌĆāSubcutaneous fat thickness | 0.14 | 0.13 | 0.13 | 0.13 | 0.01 | 0.95 | 0.60 | 0.77 | 0.99 |
| d 21 | |||||||||
| ŌĆāAbdominal fat | 1.64a | 1.55b | 1.53b | 1.53b | 0.03 | 0.04 | 0.02 | 0.83 | 0.97 |
| ŌĆāSubcutaneous fat thickness | 0.25a | 0.19b | 0.17b | 0.18b | 0.01 | 0.01 | 0.01 | 0.05 | 0.72 |
| d 28 | |||||||||
| ŌĆāAbdominal fat | 3.78 | 3.12 | 3.17 | 3.21 | 0.43 | 0.43 | 0.21 | 0.24 | 0.55 |
| ŌĆāSubcutaneous fat thickness | 0.40 | 0.36 | 0.36 | 0.36 | 0.03 | 0.53 | 0.75 | 0.41 | 0.24 |
| d 35 | |||||||||
| ŌĆāAbdominal fat | 3.76 | 3.31 | 3.18 | 3.36 | 0.29 | 0.71 | 0.49 | 0.46 | 0.99 |
| ŌĆāSubcutaneous fat thickness | 0.45 | 0.40 | 0.40 | 0.40 | 0.01 | 0.15 | 0.06 | 0.23 | 0.54 |
| d 42 | |||||||||
| ŌĆāAbdominal fat | 3.66 | 3.14 | 3.13 | 3.17 | 0.18 | 0.72 | 0.39 | 0.49 | 0.81 |
| ŌĆāSubcutaneous fat thickness | 0.49 | 0.42 | 0.42 | 0.42 | 0.02 | 0.38 | 0.19 | 0.29 | 0.61 |
Table┬Ā7
| Items | Model type | Treatment1) | Parameter2) | R2 | Inflexion point weight (g) or thickness (cm) | Maximum weekly gain (g or cm) | Inflexion point age (wk) | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| A (g/cm) | B | k (%) | |||||||
| Abdominal fat (g) | Logistic | 0FCSM | 55.25 | 248.00 | 1.26 | 0.994 | 27.63 | 17.40 | 4.38 |
| 3FCSM | 59.18 | 107.73 | 0.91 | 0.993 | 29.59 | 13.46 | 5.14 | ||
| 6FCSM | 50.88 | 106.53 | 0.94 | 0.984 | 25.44 | 11.96 | 4.97 | ||
| 9FCSM | 40.06 | 248.45 | 1.30 | 0.997 | 20.03 | 13.02 | 4.24 | ||
| Gompertz | 0FCSM | 55.00 | 24.36 | 0.81 | 0.996 | 20.22 | 16.38 | 3.94 | |
| 3FCSM | 50.00 | 24.15 | 0.80 | 0.996 | 18.38 | 14.71 | 3.98 | ||
| 6FCSM | 48.00 | 24.51 | 0.82 | 0.987 | 17.65 | 14.47 | 3.90 | ||
| 9FCSM | 45.38 | 24.28 | 0.81 | 0.996 | 16.68 | 13.51 | 3.94 | ||
| Bertalanffy | 0FCSM | 89.85 | 1.64 | 0.37 | 0.994 | 26.62 | 14.78 | 4.31 | |
| 3FCSM | 80.00 | 1.01 | 0.27 | 0.996 | 23.70 | 9.60 | 4.11 | ||
| 6FCSM | 71.42 | 1.07 | 0.26 | 0.988 | 21.16 | 8.25 | 4.49 | ||
| 9FCSM | 62.19 | 1.66 | 0.39 | 0.994 | 18.43 | 10.78 | 4.12 | ||
| Subcutaneous fat thickness (cm) | Logistic | 0FCSM | 0.50 | 35.98 | 0.93 | 0.996 | 0.25 | 0.12 | 3.85 |
| 3FCSM | 0.44 | 38.89 | 1.00 | 0.977 | 0.22 | 0.11 | 3.66 | ||
| 6FCSM | 0.45 | 36.54 | 0.91 | 0.971 | 0.23 | 0.10 | 3.95 | ||
| 9FCSM | 0.44 | 38.92 | 0.99 | 0.975 | 0.22 | 0.11 | 3.70 | ||
| Gompertz | 0FCSM | 0.54 | 6.08 | 0.46 | 0.994 | 0.20 | 0.09 | 3.92 | |
| 3FCSM | 0.48 | 6.05 | 0.45 | 0.969 | 0.18 | 0.08 | 4.00 | ||
| 6FCSM | 0.49 | 6.11 | 0.46 | 0.962 | 0.18 | 0.08 | 3.93 | ||
| 9FCSM | 0.49 | 5.94 | 0.47 | 0.968 | 0.18 | 0.08 | 3.79 | ||
| Bertalanffy | 0FCSM | 0.58 | 1.39 | 0.37 | 0.992 | 0.17 | 0.10 | 3.86 | |
| 3FCSM | 0.51 | 1.37 | 0.36 | 0.965 | 0.15 | 0.08 | 3.93 | ||
| 6FCSM | 0.53 | 1.29 | 0.36 | 0.958 | 0.16 | 0.08 | 3.76 | ||
| 9FCSM | 0.53 | 1.27 | 0.35 | 0.964 | 0.16 | 0.08 | 3.82 | ||






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





