 |
 |
| Anim Biosci > Volume 32(7); 2019 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
ACKNOWLEDGMENTS
Figure 1

Figure 2
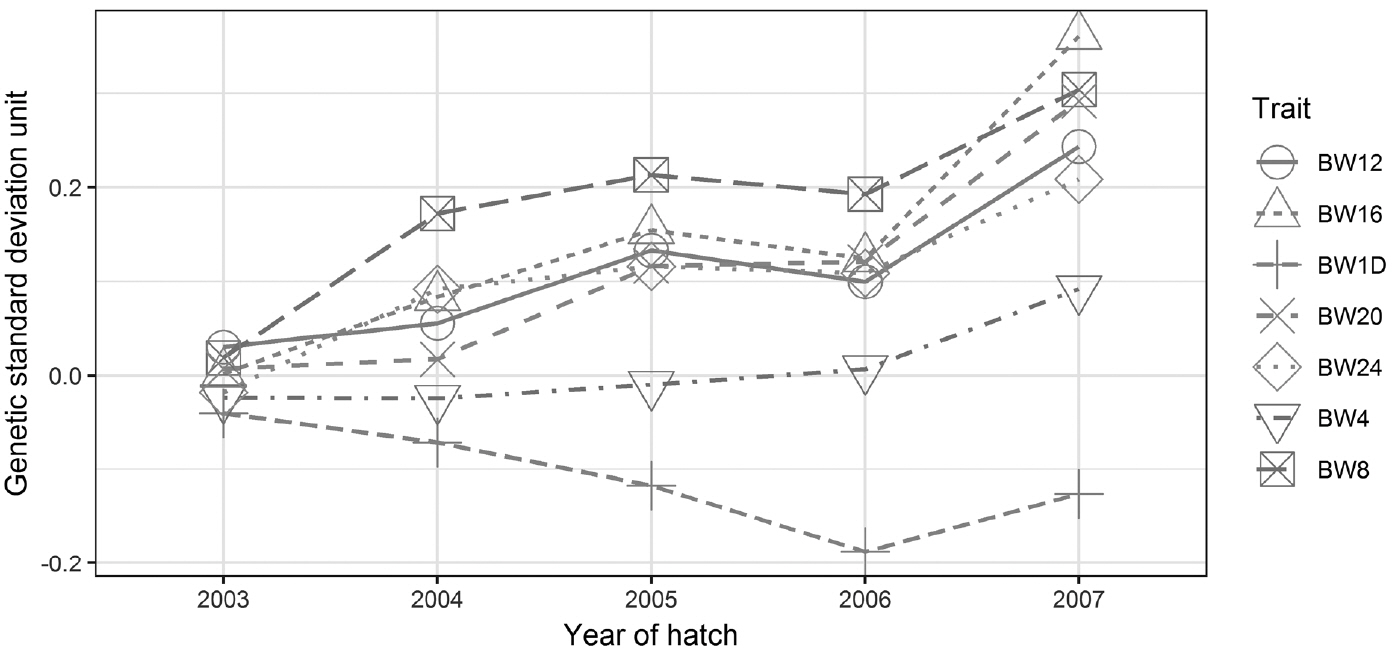
Table 1
| Traits1) | No. of records | Mean | SD | Min | Max | CV (%) |
|---|---|---|---|---|---|---|
| BW1D (g) | 11,588 | 30.93 | 3.38 | 22.00 | 40.00 | 10.93 |
| BW4 (g) | 11,201 | 218.91 | 56.68 | 46.00 | 379.00 | 25.89 |
| BW8 (g) | 10,807 | 642.08 | 138.74 | 260.00 | 1034.00 | 21.61 |
| BW12 (kg) | 9,777 | 1.10 | 0.21 | 0.52 | 1.67 | 19.09 |
| BW16 (kg) | 8,948 | 1.49 | 0.31 | 0.59 | 2.38 | 20.81 |
| BW20 (kg) | 7,643 | 1.81 | 0.41 | 0.64 | 3.00 | 22.65 |
| BW24 (kg) | 6,157 | 2.12 | 0.47 | 0.89 | 3.52 | 22.17 |
| BWFE (kg) | 1,428 | 2.05 | 0.25 | 1.40 | 2.75 | 12.20 |
| AFE (d) | 1,395 | 199.14 | 21.02 | 138.00 | 260.00 | 10.56 |
| EWFE (g) | 1,393 | 36.94 | 4.85 | 26.00 | 48.00 | 13.13 |
| EN (eggs) | 402 | 53.91 | 13.30 | 25.00 | 91.00 | 24.67 |
Table 2
| Trait1) | Model2) | σ2a | σ2m | σ2pe | σ2p | h2a | h2m | c2pe |
|---|---|---|---|---|---|---|---|---|
| BW1D (g) | D | 1.20±0.23 | 2.96±0.51 | 3.13±0.38 | 11.51±0.33 | 0.10±0.02 | 0.26±0.04 | 0.27±0.03 |
| BW4 (g) | D | 232.65±32.31 | 47.98±19.11 | 91.40±17.03 | 1,141.78±20.34 | 0.20±0.03 | 0.04±0.02 | 0.08±0.02 |
| BW8 (g) | D | 2,645.90±264.98 | 301.01±130.00 | 379.14±104.73 | 7,860.71±153.07 | 0.34±0.03 | 0.04±0.02 | 0.05±0.01 |
| BW12 (kg) | D | 0.01±0.00 | 0.00±0.00 | 0.00±0.00 | 0.02±0.00 | 0.37±0.03 | 0.03±0.02 | 0.05±0.01 |
| BW16 (kg) | D | 0.01±0.00 | 0.00±0.00 | 0.00±0.00 | 0.04±0.00 | 0.30±0.03 | 0.04±0.02 | 0.04±0.01 |
| BW20 (kg) | D | 0.02±0.00 | 0.00±0.00 | 0.00±0.00 | 0.06±0.00 | 0.30±0.03 | 0.02±0.02 | 0.06±0.02 |
| BW24 (kg) | B | 0.03±0.00 | 0.01±0.00 | - | 0.09±0.00 | 0.30±0.04 | 0.10±0.02 | - |
| BWFE (kg) | A | 0.03±0.00 | - | - | 0.05±0.00 | 0.47±0.06 | - | - |
| AFE (day) | C | 40.49±14.74 | - | 13.95±7.58 | 246.37±10.04 | 0.16±0.06 | - | 0.06±0.03 |
| EWFE (g) | A | 3.03±0.91 | - | - | 19.40±0.77 | 0.16±0.05 | - | - |
| EN (egg) | A | 24.50±17.52 | - | - | 161.65±12.00 | 0.15±0.11 | - | - |
LHKK, Lueng Hang Kao Kabinburi; σ2a, direct additive genetic effect; σ2m, maternal genetic effect; σ2pe, permanent environmental hen effects.
Table 3
LHKK, Lueng Hang Kao Kabinburi; BW1D, body weight at day 1; BW4, BW8, BW12, BW16, BW20, BW24 are body weight at 4, 8, 12, 16, 20 and 24 weeks of age, respectively; BWFE, body weight at first egg; AFE, age at first egg; EWFE, egg weight at first egg; EN, total number of eggs laid from onset of lay to 17 weeks of lay.
Table 4
LHKK, Lueng Hang Kao Kabinburi; BW1D, body weight at day 1; BW4, BW8, BW12, BW16, BW20, BW24 are body weight at 4, 8, 12, 16, 20 and 24 weeks of age, respectively; BWFE, body weight at first egg; AFE, age at first egg; EWFE, egg weight at first egg; EN, total number of eggs laid from onset of lay to 17 weeks of lay.
Table 5
| Trait1) | Total no. of birds | No. of inbred birds (%) | Average inbreeding coefficients2) | SD | Regression coefficient3)±SE | Inbreeding effect on trait mean (% of mean) | p-value |
|---|---|---|---|---|---|---|---|
| BW1D (g) | 11,588 | 733 (6.33) | 0.24 | 1.45 | −0.090±0.02 | −0.29 | <0.0001 |
| BW4 (g) | 11,201 | 726 (6.48) | 0.24 | 1.45 | −0.320±0.24 | −0.15 | 0.26ns |
| BW8 (g) | 10,807 | 721 (6.67) | 0.24 | 1.43 | 0.630±0.63 | 0.10 | 0.48ns |
| BW12 (kg) | 9,777 | 705 (7.21) | 0.26 | 1.46 | 0.003±0.00 | 0.27 | 0.02* |
| BW16 (kg) | 8,948 | 661 (7.39) | 0.26 | 1.43 | 0.001±0.00 | 0.07 | 0.05ns |
| BW20 (kg) | 7,643 | 580 (7.59) | 0.25 | 1.41 | 0.002±0.00 | 0.11 | 0.39ns |
| BW24 (kg) | 6,157 | 497 (8.07) | 0.24 | 1.26 | 0.004±0.00 | 0.19 | 0.34ns |
REFERENCES
- TOOLS
-
METRICS

- Related articles
-
Genetic parameter analysis of reproductive traits in Large White pigs2022 November;35(11)
Genetic diversity and population genetic structure of Cambodian indigenous chickens2022 June;35(6)
Genetic parameters and litter trait trends of Danish pigs in South Vietnam2021 December;34(12)






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print




