MATERIALS AND METHODS
Animal care
The experimental procedure was approved by the Institutional Animal Care and Use Committee at Universidade Federal de ViûÏosa (19/2017).
Experimental area
The experiment was carried out at the Department of Animal Science of the Universidade Federal de ViûÏosa, MG, Brazil, over the dry season, the dry-to-rainy transition season, and the rainy season. The climate of the area was classified according to KûÑppen-Geiger [
7] as Cwa (humid temperate, with dry winter, hot summer).
Animal management and experimental treatments
Twenty-four pregnant Nellore primiparous cows with an average body weight (BW) of 409ôÝ8.0 kg, 22ôÝ0.9 mo old, and at 172ôÝ2.5 d of gestation were used. The animals were managed on an experimental area of Brachiaria decumbens pastures divided into six paddocks of 4-ha for grazing with continuous stocking. Cows had unlimited access to water and mineral mix (87 g/kg calcium, 90 g/kg phosphor, 187 g/kg sodium, 90 g/kg sulphur, 2,400 mg/kg zinc, 800 mg/kg copper, 1,600 mg/kg manganese, 40 mg/kg iodine, 8 mg/kg cobalt, and 8.16 mg/kg selenium).
The experimental treatments were control (no concentrate supplement was fed); daily supplementation (1.5 kg/d/cow of supplement); and infrequent supplementation (4.5 kg/cow of supplement every three days; formulation of the concentrate supplement was outlined to avoid any potential acidosis [
8]. The energy-protein supplement on an as-fed basis was composed of 425 g/kg wheat meal, 213 g/kg corn meal, 332 g/kg soybean meal, 27 g/kg urea, and 3 g/kg ammonium sulphate. The chemical composition of the experimental supplement is shown in
Table 1.
A quantity of supplement (1.5 kg/d/cow) containing 450 g of crude protein (CP) was chosen to meet the protein requirements of primiparous cows, with an average gain of 0.6 kg/d at pasture with approximately 60 g/kg CP (dry matter [DM] basis) in order to produce mature weight female zebu cattle according to recommendations of Nutrient Requirements of Zebu and Crossbred Cattle (BR-CORTE) [
9] upon parturition.
Animal data collection
The animals were weighed at the beginning of the trial (105 d before calving), 15 d before parturition, at calving, and at the end of the trial (105 d after calving). Calf BW was recorded at birth. The BWs were obtained at were at 0600 h, except on the day of parturition. Calf BW was also recorded at birth and at 105 d after parturition. Upon analysis the BW were corrected to shrunk BW [
10] in order to avoid possible confounding effect of digest:
Where, SBW is the shrunk body weight (kg) and BW is the body weight (kg).
Ribeye area (RA), fat thickness over the longissimus dorsi (between the 12th and the 13th ribs) and fat thickness over the biceps femoris muscle were recorded with an ultrasound (Aloka SSD 500; 3.5 MHz linear probe; Aloka Co. Ltd., Wallingford, CT, USA). Images were analysed in the BioSoft Toolbox II for Beef software (Biotronics Inc., Ames, Iowa, USA). In the morning of the same day that the ultrasound was performed, the body condition score (BCS) was recorded by three experienced technicians on a scale ranging from 1 to 9.
Feed sampling and chemical analysis
Representative samples of supplement were collected monthly. Pasture sample compositions were obtained by hand-clipping every two weeks. Once a month, a second pasture sample was collected from each paddock. The second samples consisted of four forage subsamples randomly selected using a metal (0.5û0.5 m) square; the pasture was clipped approximately 1 cm above the ground to estimate the potentially digestible forage dry matter (pdDM) availability according to Paulino et al [
11]. Samples of supplement and pasture were oven-dried (55ô¯C) and ground in a Wiley mill (model 3; Arthur H. Thomas, Philadelphia, PA, USA) to pass through a 2-mm screen. Half of each ground sample was ground again to pass through a 1-mm screen.
The pdDM was estimated using the second pasture sam ple collected in each period as previously described, using the following equation [
11]:
where 0.98 represents the true digestibility coefficient of the forage cell content; NDF represents the neutral detergent fiber assayed with a heat stable amylase; and iNDF is the forage content of indigestible neutral detergent fiber.
Two 9-d intake-digestibility trials were performed through out the experimental period, the first at 55 d before parturition and the second at 55 d after parturition. Titanium dioxide (TiO
2) was used as an external marker to estimate fecal excretion (FE) [
12]. Twenty grams of TiO
2 per animal was packaged in paper cartridges and delivered via the oesophagus with a metal probe, once daily at 1030 h over nine days. Six days were allowed for stabilization of the external marker excretion, and fecal samples were collected at 0800 h and 1500 h on the seventh day, at 1000 h and 1700 h on the eighth day, and at 0600 h and 1300 h on the ninth day of the intake trial. Approximately 300 g of fecal sample was collected immediately after spontaneous defecation. Each fecal sample was oven-dried (55ô¯C) and ground as described for pasture. Ground samples were proportionally mixed to make a single representative sample per animal on the pre- and postpartum.
Pooled samples of each material ground through 1-mm screen (supplement, pasture, and feces) were analysed according to the standard analytical procedures of the Brazilian National Institute of Science and Technology in Animal Science (INCT-CA; [
13]; for DM (dried overnight at 105ô¯C; method INCT-CA number G-003/1), ash (complete combustion in a muffle furnace at 600ô¯C for 4 h; method INCT-CA number M-001/1), N (Kjeldahl procedure; method INCT-CA number N-001/1), ether extract (method INCT-CA number G-004/1), and NDF corrected for ash and protein (apNDF, using a heat-stable öÝ-amylase, omitting sodium sulphite and correcting for residual ash and protein; method INCT-CA number F-002/1). The fecal samples were also analysed for levels of TiO
2 by colorimetric (method INCT-CA M-007/1). From samples of supplement, pasture, and feces processed through a 2-mm screen, the iNDF content was determined as the residual NDF remaining after 288 h of ruminal
in situ incubation using F57 filter bags (Ankom Technology Corp., Macedon, NY, USA), according to Valente et al [
14].
The FE was estimated by the ratio of TiO 2 and its concentration in the feces. The DM intake was estimated by using the iNDF as an internal marker and calculated by the following equation:
where FE is the fecal excretion (kg/d); iNDFfeces is the concentration of iNDF in the feces (kg/kg); iNDFsup is the iNDF in the supplement (kg); iNDFforage is the concentration of iNDF in forage (kg/kg); and DMSI is the DM supplement intake (kg).
Blood hormone and metabolite assessment
Blood samples were collected in the peripartum period, critical period of physiological changes, by puncture of the jugular vein only at one single day, after 3 days of the infrequent supplementation and before the next infrequent supplementation at 0700 h at 27 d and 9 d prior to parturition, at the calving day, 9 d and at 27 d after parturition. Blood was collected into vacutainers with gel for serum separation and clot activation (BD Vacuntainer SST II Plus, SûÈo Paulo, Brazil) for analyses of insulin-like growth factor-1 (IGF-1), non-esterified fatty acids (NEFA), öý-hydroxybutyrate (öý-OHB), cholesterol, triglycerides, total protein, albumin, and urea. A second blood sample was collected in a second tube with ethylenediamine tetraacetic acid (EDTA) and sodium fluoride (BD Vacutainer Fluoreto/EDTA, SûÈo Paulo, Brazil) for glucose analysis. Both tubes were centrifuged at 2,700ûg for 20 min. Following centrifugation, the plasma and serum were collected and subsequently frozen at ã20ô¯C for further analysis. Immediately after the centrifugation, a sample of plasma was collected to assess the concentration of free amino acids (AA).
Serum concentrations of IGF-1 were analysed by chemi luminescence using a Liaison analyser and Diasorin kit (DiaSorin, Saluggia, Italy). The levels of NEFA were quantified by the colorimetric method, and öý-OHB was analysed by the kinetic enzymatic method based on the oxidation of D-3-hydroxybutyrate to acetoacetate (Ref. Numbers FA115 and RB1007 respectively, Randox, Ireland, UK). The concentration of free AA in serum was obtained using the high-performance liquid chromatography techniques described by Pitta et al [
15]. Glucose (K082, Bioclin Quibasa, Belo Horizonte, Brazil), cholesterol (K083, Bioclin Quibasa, Brazil), triglycerides (K117, Bioclin Quibasa, Brazil), and urea (K056, Bioclin Quibasa, Brazil) were quantified by the enzymatic-colorimetric method and total protein (K031, Bioclin Quibasa, Brazil); albumin (K040, Bioclin Quibasa, Brazil) was analysed by the colorimetric method. All the analyses previously mentioned were determined by an automated biochemical analyser (Mindray BS 200E, Shenzhen, China). Globulins were calculated by subtracting the albumin quantified from the total protein level.
Hepatic tissue and skeletal muscle biopsy
Biopsies of hepatic and skeletal muscle tissue were performed on the 27th day prior to calving. Six animals from each treatment were randomly selected for biopsies.
Liver sampling was performed via needle biopsy (Tru-Cut biopsy needle; Care Fusion Corporation, San Diego, CA, USA) 4 h before supplement feeding according to the procedure described by Mû¡lgaard et al [
16]. The incision was made between the 11th and 12th ribs from the right hepatic lobe [
17]. Skeletal muscle sampling was performed on the left side at the 13th rib, three-fifths of the distance from the medial to the lateral edge of the longissimus muscle. Immediately, the liver samples (100 mg of tissue) and skeletal muscle samples (1 cm
3) were placed in cryotubes, frozen and stored in liquid nitrogen at ã196ô¯C until processing.
Abundance of carbamoyl phosphate synthase and mRNA expression of skeletal muscle energy metabolism markers
Whole liver protein was extracted in lysis buffer (10 mM Tris, pH 7.2; 0.5% Triton X-100; 10% glycerol; 0.5% dithiothreitol; 0.5 mM phenylmethanesulfonyl fluoride and 0.5 mM benzamidine). The protein content was measured with the Bradford Protein Assay (Bio-Rad, Hercules, CA, USA), and an equal amount of protein was separated with a 10% dodecyl sulphate-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes and treated with blocking solution (3% bovine serum albumin w/v in tris-buffered saline with triton-X100 solution - TBSt) for 1 h with gentle agitation at room temperature. Membranes were then incubated with the following primary antibodies against carbamoyl phosphate synthase-1 (CPS-1 no. SC-376190, Santa Cruz, Dallas, TX, USA). The primary antibody was incubated at a 1:500 dilution in the blocking solution for 16 h at 4ô¯C with gentle agitation. After incubation with the primary antibody, the membranes were washed 3 times at room temperature with TBSt and then incubated with the appropriate horseradish peroxidase secondary antibody (goat anti-mouse) at 1:5,000 dilution, for 1 h at room temperature with gentle agitation. Then, the membranes were washed 3 times (5 min each) with TBSt, developed with Clarity TM ECL substrate (Bio-Rad, USA), scanned with a c-Digit Blot scanner, and analysed with Image Studio (LI-COR Inc., Lincoln, NE, USA). The band density of target proteins was normalized using the density of bands of the load control samples that were handled and loaded under the same conditions as the target samples.
Total RNA (1 ö¥g) was extracted from 0.5 g of muscle tissue samples using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The RNA integrity (RIN) was evaluated by capillary electrophoresis using an RNA 6000 Nano kit and a 2100 Bioanalyser System (Agilent Techonologies, Santa Clara, CA, USA). Samples with RIN >7.0 were treated with DNAse I, Amplification Grade (Invitrogen, USA) and reverse transcribed into cDNA using the GoScript Reverse Transcription System (Promega, Madison, WI, USA). The
mRNA levels of carnitine palmitoyl transferase 1 (
CPT-1) were quantified using the following primers: Forward - GTCCCTTCCCTTGCTCTA, Reverse - GGACAGCAGAGACCCATA, while the
mRNA expression of peroxisome proliferator-activated receptor ö° coactivator 1 öÝ (
PGC-1öÝ) was quantified using the following primers: Forward -GAAGCGGGAATCCGAAAG, Reverse - CTCAGTT CTGTCCGTGTTG. The housekeeping gene used was 18S, which was quantified using the following primer: Forward - CCTGCGGCTTAATTTGACTC, Reverse - AACTAAGAA CGGCCATGCAC. A quantitative polymerase chain reaction (qPCR) was performed on a 7300 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) using a GoTaq kit (Promega, USA) and the following cycle parameters: 95ô¯C for 3 min and 40 cycles at 95ô¯C for 10 s and 60ô¯C for 30 s. The amplification efficiency ranged from 0.90 to 0.99. After amplification, a melting curve (0.01ô¯C/s) was used to confirm product purity. Relative gene expression data was calculated as described by Livak and Schmittgen [
18].
Statistical analysis
Statistical analyses were performed using PROC MIXED in SAS 9.4 (SAS Inst., Cary, NC, USA) and analysed according to a completely randomized design. The groups of animals were considered the experimental units, as per the following statistical model:
where Yijk is the observation taken on subject k in experimental unit j undergoing treatment I; ö¥ is the overall constant; Ti is the effect of treatment i (fixed effect); G(i)j is the effect of the group nested to the treatment i (random effect); and ã(ij)k is the unobservable random error associated with each observation.
Contrasts were constructed in order to evaluate the effects of supplementation (contrast between cows supplemented daily + cows infrequently supplemented vs non-supplemented cows) and frequency of supplementation (contrast between cows supplemented daily versus cows supplemented every three days). Due to the high probability of type II error, öÝ = 0.10 was adopted. Initial BW and BCS were used as covariates. The choice of the best (co)variance matrix was performed following the Akaike information criteria with correction. The degrees of freedom were estimated according to the Kenward-Roger method. The blood IGF-1 and metabolites variables were evaluated as repeated measures over time [
19].
DISCUSSION
The forage mass available was not a limiting factor of feed intake in this study (
Table 1). The interpretation of forage available for grazing as a baseline nutritional resource should be conducted from the perspective of the fraction potentially convertible into animal product; this can be achieved through utilization of pdDM which integrates the quantity and quality regardless of season [
11]. The overall average pdDM mass at prepartum (81 g/kg BW) and at postpartum period (89 g/kg BW) were higher than 40 to 50 g/kg BW for satisfactory intake and performance in a grazing system [
20]; thus throughout the duration of the trial, the animals had the possibility of highly selective grazing and choosing the best-quality forage parts.
Although the provision of supplemental nitrogen has been reported to increase DM intake substantially [
7], this pattern was not observed (
Table 2). The adequacy of the dietary protein-to-energy ratio has been pointed out as one of the main indicators of the intake patterns of cattle fed tropical forages [
21]. The maximum forage intake has been observed with dietary CP:dOM at approximately 216 g/kg [
22]. Although there is a higher dietary CP:dOM for supplemented cows than non-supplemented cows during the pre- and postpartum periods (
Table 2), the dietary CP:dOM observed in our study for supplemented cows was below the value suggested by Reis et al [
22]. Thus, regardless of the treatment, all cows had low dietary protein-to-energy ratios (CP:dOM <216 g/kg) which seems to support an unaltered forage intake between supplemented and non-supplemented cows as well as between cows from different supplementation frequencies. However, similar FDM intake between cows supplemented daily and cows supplemented every three days is an indicator that the reduction of supplementation frequency can be attractive to cow-calf producers. Usually, infrequent supplementation under medium-high quality forage has been reported as a reduction in the forage voluntary intake [
23].
Energy-protein supplementation increased the CP intake for supplemented cows due to the additional supply of protein provided by the supplement (
Table 2). During the prepartum period, supplemented cows had a greater OM and CP digestibility compared to non-supplemented cows (
Table 3), which resulted in a greater dOM intake for supplemented cows (
Table 2). Such a pattern has been associated with the supplementation of the animals, since concentrates usually have a greater digestibility than forage [
24]. However, during the postpartum period, the supplementation increased only the CP digestibility (
Table 3). On the other hand, the reduction of supplementation frequency did not change intake (
Table 2) and total apparent digestibility (
Table 3). Such observations may be explained by the fact that the same quantity of supplement was offered to both treatments (daily and infrequent). Previous studies have demonstrated that cattle can efficiently recycle urea to supply nitrogen to the rumen [
25,
26]. Thus, it is possible that animals from the infrequent group may have increased the urea recycling and kept the levels of nitrogen in the rumen at equivalent levels of daily supplemented animals, which may have contributed for a lack of change in forage intake between daily and infrequently supplemented animals.
The increase in dOM and CP intake increased the BW, ADG during the prepartum period (15 d before calving;
Table 4), and adjBW at parturition (
Table 4) for supplemented cows. Primiparous cows seem to be more sensitive to nutrient intake and consequently BCS changes more drastically than non-primiparous animals [
8], but this pattern was not observed. In fact, regardless of treatment, all cows had a BCS between the minimum (5.0) and maximum (6.0) at calving which are acceptable values according to the NASEM [
8] recommendation to allow the reproductive success of the animals during the breeding season. Our data suggest that non-supplemented cows may adapt their energy metabolism (e.g., maintenance energy) to periods of lower availability of nutrients [
27] allowing them to maintain similar BCS, RA, and fat-thickness compared to supplemented cows on prepartum (
Table 4). During the postpartum period, there was no significant dOM effect which ultimately produced similar BW, BCS, RA, FAT-Ld, FAT-Bf, and ADG between supplemented and non-supplemented cows (
Table 4). Intake during the pre- and postpartum periods in cows supplemented daily and cows supplemented every three days was not significantly different (
Table 2) and resulted in similar BW, adjBW, calvingBW, ADG, BCS, RA, FAT-Ld, FAT-Bf, and ADG (
Table 4).
Maternal supplementation during the last trimester of pregnancy has been reported to be an important factor for fetal growth, altering calf birth weight [
8]. However, in our study, no over- and underfeeding was observed among cows subjected to different strategies of supplementation (
Table 2). Thus, similar calf BW for all treatments was observed (
Table 4).
Nutrient intake pre- and postpartum influences concen trations of IGF-1 in serum of primiparous lactating beef cows [
28]. Reduced nutrient intake uncouples the growth hormone (GH)ãIGF-1 axis and it increases GH secretion in cattle; whereas serum concentrations of IGF-1 are decreased. The decline in circulating IGF-1 is paralleled by a decline in circulating insulin that stimulate hepatic gluconeogenesis providing glucose for the fetus or for lactose synthesis; additionally, NEFA is mobilized from adipose tissue to provide energy for peripheral needs [
29]. Elevated NEFA levels can result in ketone body production, such as öý-OHB, another energetic substrate [
30]. Thus, the data presented by this current study demonstrate that the intake of nutrients through the peripartum period was enough to avoid problems concerning energy balance between supplemented and non-supplemented cows and between cows supplemented daily and cows supplemented every three days. The similar NEFA and öý-OHB levels between non-supplemented and supplemented cows and between cows subjected to different supplementation strategies support such an argument (
Table 5). Furthermore, NEFA levels during the peripartum period (
Table 5;
Supplementary Figure S2a) were below the threshold of 0.40 mmol/L, the value utilized by Lopes et al [
31] as an indicative of problems with energy balance in grazing beef cows.
Cholesterol and triglyceride levels between supplemented and non-supplemented cows and between the studied frequency treatments were not different (
Table 5). Thus, our data indicate that cows in all treatments were able to cope with the öý-oxidation of NEFA and to export those not used as metabolic fuel.
The dietary intake of starch substrates is directly associated with greater hepatic gluconeogenesis in ruminants [
32]. However, cows subjected to decreased supplementation frequency had low concentrations of glucose (
Table 5). Since blood sampling occurred only on one single day, 3 days after infrequent supplementation and before the next supplementation, it is possible that plasma glucose concentrations decreased during the days that no supplementation was offered. Thus, no effects of supplementation on the plasma concentration of glucose between supplemented and non-supplemented cows were observed due the large variance in glucose levels between cows of different supplementation frequencies (
Table 5).
Cows in a negative energy state increase body fat mobili zation followed by a high expression of the genes involved in fatty acid oxidation and utilization, such as
CPT-1 and
PGC-1öÝ [
33,
34]. Therefore, our data indicate a lack of effects on
CPT-1 and
PGC-1öÝ mRNA expression in skeletal muscle (
Table 6) which suggests that the energy metabolism of skeletal muscle did not changed between treatments, which may explain the similar NEFA levels between supplemented and non-supplemented cows and between cows subjected to different supplementation frequencies.
A dependence on nitrogen recycling in the rumen is an indication of dietary protein deficiency [
21]. This biological event can be significant even in medium-high quality forage and is evidenced by low SUN levels due to transfer of forage to the rumen to maintain microbial growth [
5]. Furthermore, situations where nitrogen recycling occurs without myofibrillar protein mobilization [
5,
35], associated with a predominant use of absorbed AA for nitrogen recycling, may suggest a low peripheral circulation pool of free AA. Thus, at 27 and 9 d prepartum and at calving day, supplemented cows had higher SUN levels than non-supplemented cows (
Figure 1b) due to the increase in dietary nitrogen intake associated with higher rates of ammonia transfer from the rumen, output of urea from the liver into the blood, and lower SUN transfer into the rumen [
24]. However, the pool of free AA was higher only at 9 d before calving (
Figure 1a) for supplemented cows. Thus, it is reasonable to believe that supplemented cows had enough escape of dietary protein to avoid the use of metabolizable AA for the synthesis of urea for recycling at 9 d before calving.
Lactation peaks in Nellore cows occur between the third and fourth postpartum week [
9]. Thus, the higher blood AA at 27 d postpartum (
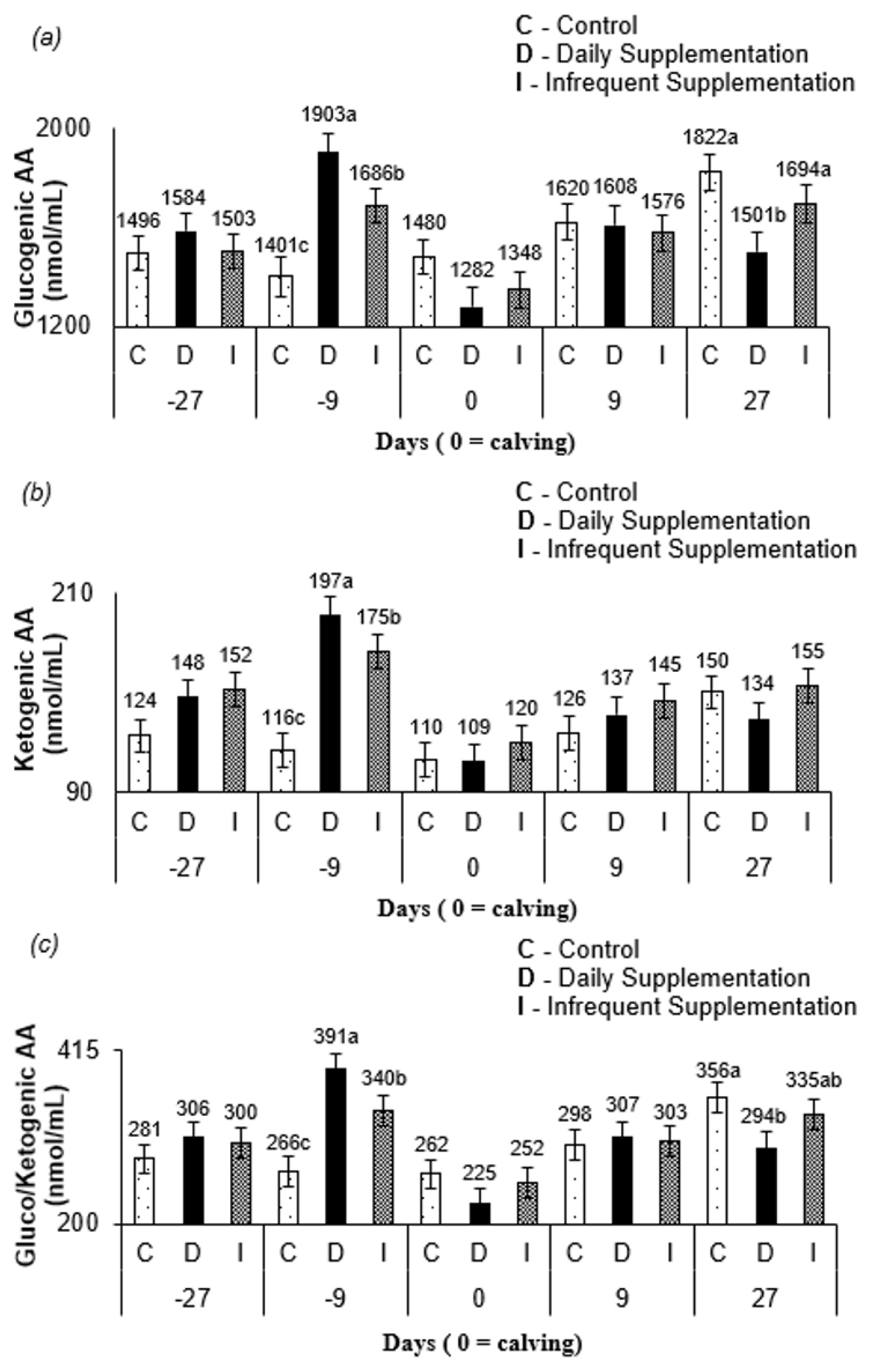
Figure 1a) for cows from control and infrequent treatment compared to that in cows from daily treatment occurred to support milk production, since these treatments did not have a daily constant availability of energy and protein compounds via supplement. The greater amount of glucogenic AA (
Figure 2a), a precursor of lactose synthesis for cows, from control and infrequent treatment at 27 d after parturition corroborates this conclusion. Furthermore, since the blood sampling occurred on the third day of the supplementation cycle, the AA mobilization likely increased through the days when no supplementation was offered for infrequently supplemented cows. The levels of glucogenic AA, ketogenic AA, and gluco/ketogenic AA (
Figure 2c) for all treatments at 9 d prepartum and at 27 d postpartum may have occurred due to homeostatic AA concentration control [
24]. The levels of the free AA pool pattern in all treatments (
Figure 1a) are consistent with this argument.
Protein supplementation frequency and protein over- and underfeeding have previously been explored scientifically and reported with a greater action of enzymes involved in the urea cycle, such as CPS-1 [
36,
37]. The CP intake (
Table 2) of non-supplemented cows was above the minimum level required to meet their maintenance requirements [
9]. Likewise, daily supplemented cows had CP intakes below the threshold maximum at which positive responses to supplemental protein have been observed (225 g CP/kg DM) [
21]. Thus, this may suggest that the similar values for hepatic CPS-1 abundance (
Table 6) observed between supplemented and non-supplemented cows occurred because dietary protein was not extreme enough to alter CPS-1 abundance. This can explain the similar CPS-1 abundance between cows from different supplementation frequencies, since for the infrequent supplementation, the CP intake was approximately 200 g CP/kg DM (
Table 2).
In summary, our data indicate that the reduction of sup plementation frequency (4.5 kg/cow of supplement every three days) does not negatively affect the performance and metabolic characteristics of primiparous cows under grazing conditions. Moreover, the results suggest that energy-protein supplementation during the pre- and postpartum periods of primiparous beef cows under grazing conditions likely decreased forage intake leading to a similar nutrients intake among treatments and no changes on animal performance.








 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print





