INTRODUCTION
Lutein is a xanthophyll carotenoid with an orange color, rich in some plants [
1]. Commercially, marigold flowers are a primary source of lutein, and the marigold lutein is often used as a coloring agent in food and feed [
2]. Besides that, lutein as an eye pigment is essential for humans and animals that are unable to synthesize [
3]. More importantly, lutein is a critical antioxidant for plants and animals [
4]. Lutein in the body tissues was shown to have beneficial effects on the visual, nervous, cardiovascular, urinary, and immune systems [
5,
6]. Several latest studies reported that lutein improved oocytes, embryo quality, fertilization rate, and 2-cell blastocyst in the polycystic ovary syndrome model of mice [
7,
8]. Lutein increased cumulus expansion, oocyte viability, and embryo developmental potential to the morula stage by protecting lipid components in oocytes and embryos against oxidative injury in swine [
9]. Additionally, during egg incubation, the antibacterial capacity of egg lysozyme was highly dependent on yolk lutein content [
10]. However, studies showed that dietary lutein added at 10 to 40 mg/kg of diet had no significant effect on the egg production of hens [
11,
12].
The findings related to reproduction raise the possibility that lutein can be vertically transmitted from mother to offspring. The vertical transmission may be necessary in improving the fertility and hatchability of breeders, but data are scarce. Additionally, aged broiler breeder hens have poorer egg production and fertility than other species, which is a technical constraint in practice. Based on the knowledge between lutein and reproduction, the present study aimed to test the hypothesis that supplementing lutein to the diet can improve follicular development, egg production, fertility, hatchability, and reproductive hormones, and decrease oxidative injury in aged broiler hens.
MATERIALS AND METHODS
Animal ethics approval
Research on animals was approved by institutional committee on animal use in Henan University of Science and Technology (No. 2021002).
Lutein and diets
Lutein was extracted from marigold flowers. A basal diet (control, CON) contained white corn and soybean meal as main ingredients. The lutein was added at 25 (L1), 50 (L2), and 75 mg/kg (L3) of diet using step by step enlargement method. The determined lutein contents in the CON, L1, L2, and L3 were 0.26, 24.37, 49.02, and 74.28 mg/kg, respectively. The basal diet was formulated referring to the nutrient requirements of Arbor Acres parent stock Handbook (Aviagen, 2018). The ingredients, chemical levels, and lutein contents in diets are listed in
Table 1.
Animals and samples
A total of 576 Arbor Acres parent stock hens at 60 wk of age without statistical differences in egg production and body weights were randomly assigned to four dietary groups. Each treatment contained six replicates of 24 hens in two-tiers cages. All replicates were uniformly distributed in a closed chicken house to minimize the environmental effect [
13]. All hens were supplied with feed at 135 g/hen/d and
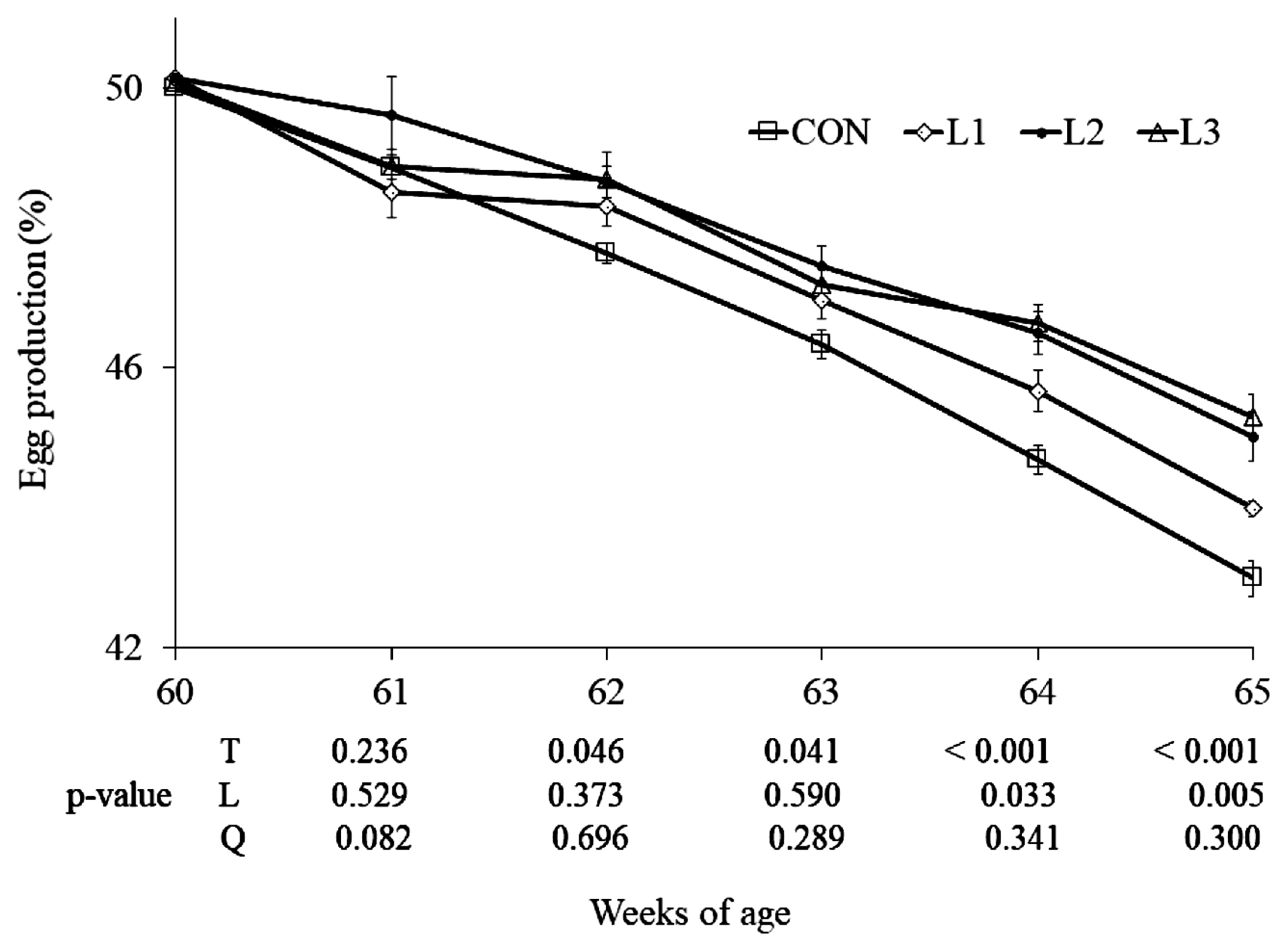
ad libitum water in the house with auto-ventilation, lighting system 16L/8D (light/dark) and temperature 20°C according to the management manual of Arbor Acres parent stock hens. After a week of adjustment, the feeding trial lasted from 61 to 65 wk of age. Artificial insemination was administered once every five days. Eggs and mortality per replicate were recorded daily and egg laying rate was calculated weekly [
14]. Hen’s health was monitored twice daily.
At the last trial day, eight hens per replicate were randomly selected and wing vein blood was collected to analyze hormones, oxidative products, and lutein content [
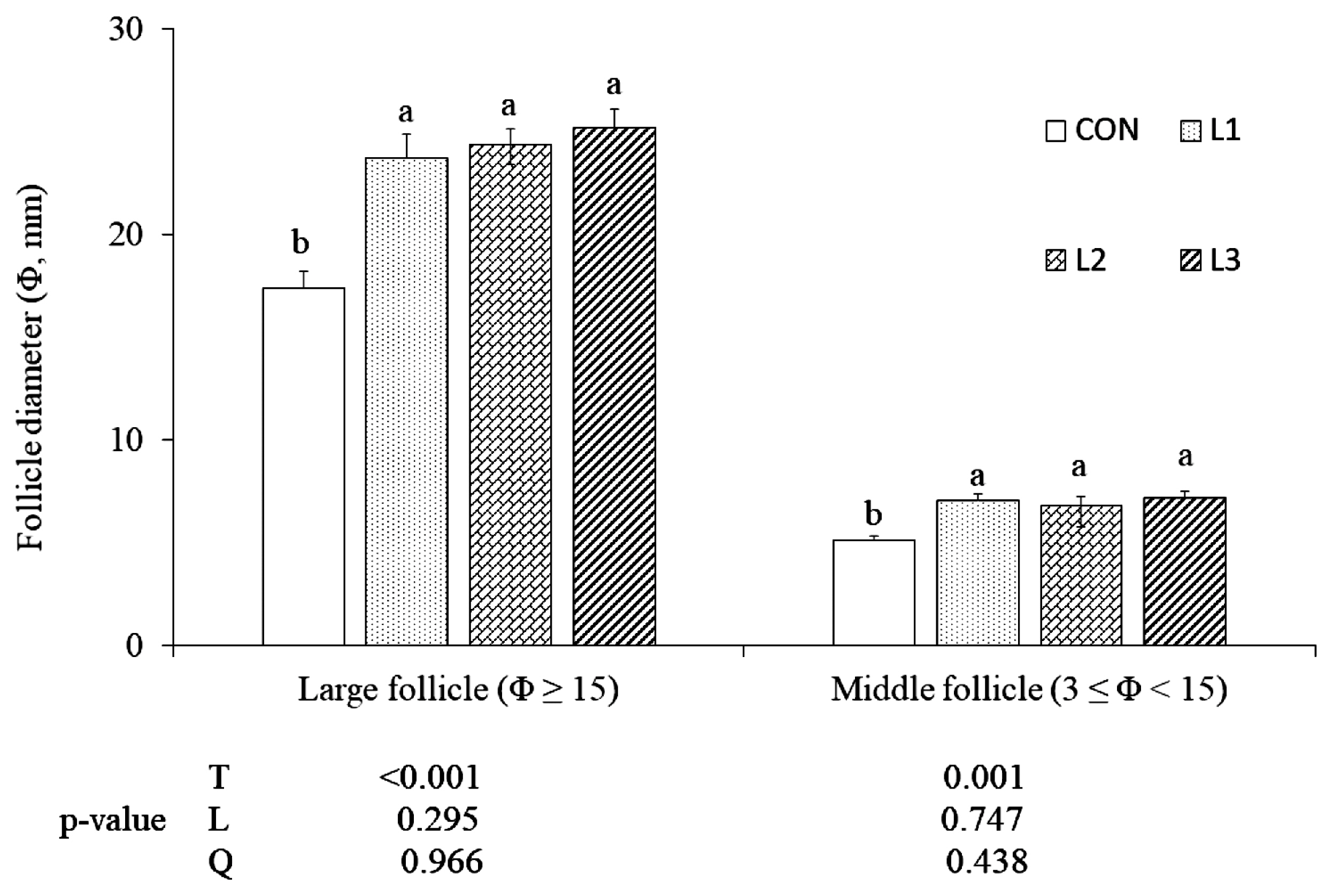
15]. The eight hens were euthanized by carbon dioxide and dissected, and follicular diameters were measured at two grades, large follicles (Φ≥15 mm) and middle follicles (3≤Φ<15 mm). At the last week of feeding trial, 300 eggs per replicate were incubated to determine fertility and hatchability. At day 7 of incubation, candling inspection was carried out to calculate the fertility (%, fertilized/total hatched eggs). At day 21, chicks were sorted out and hatching rate (%, chicks/total fertilized eggs) were calculated. A total eight yolks per replicate were used to determine lutein and oxidative product content.
Chemical and biochemical analysis
Lutein content in the marigold product and diets were measured by high performance liquid chromatography equipment (Agilent 1260; Agilent Technologies, Santa Clara, CA, USA) according to the method of China National Standard GB 26405-2011. The detection conditions were set as detector wavelength 446 nm, silica gel column 4.6 mm×250 mm (size, 3 μm), the ratio of hexane to ethyl acetate 70/30 (v/v), flow rate 1.5 mL/min, and injection volume 10 μL at room temperature. The spike recovery of serially diluted lutein was 96.2% to 105.7%.
The concentrations of hormones and oxidative products were measured using commercial kits from Nanjing Jiancheng Biological Institute (Nanjing, China) for estradiol (E2; H102-1), follicle-stimulating hormone (FSH; H101-1-2), luteinizing hormone (LH; H206-1-2), prolactin (PRL; H095-1-2), progesterone (PROG; H089), malondialdehyde (MDA; No. A003), protein carbonyl (PCO; No. A087), and 8-hydroxy 2 deoxyguanosine (8-OHdG, No. H165). The unit of MDA, PCO, and 8-OHdG were expressed as per gram protein in the yolk samples to minimize the errors during sample preparation [
16]. During the chemical and biochemical analysis, all samples were determined in triplicate.
Statistical analysis
Data are represented as mean and SEM using SPSS software (version 23; IBM SPSS, Armonk, NY, USA). Differences between mean values of normally distributed data were assessed with one-way analysis of variance (Tukey test) at p<0.05 level of significance. The statistical unit was the mean of 24 hens for egg production, of eight hens for follicles, hormones, and oxidative injury indexes in the serum, of 300 eggs for fertility and hatchability, and of eight yolks for lutein contents and oxidative injury indexes. The variable responses to the lutein doses of 25, 50, and 75 mg/kg were analyzed using contrasts of linear and quadratic polynomials.
DISCUSSION
Lutein is synthesized by plants and is an important antioxidant in nature. Currently, the egg industry uses dietary lutein to vary yolk color according to consumer preference. Besides that, ingested lutein circulates and is deposited in the body to exert an antioxidative effect in human and animal tissues. The antioxidative effect of lutein may be helpful to the egg production of hens, especially aged hens. Indeed, in the present study, the breeder hens fed with lutein at 25, 50, or 75 mg/kg had a higher laying rate than CON. However, this is inconsistent with the report that dietary lutein at 10, 20, 30, and 40 mg/kg had no significant effect on egg mass, feed intake, and egg weight of laying hens at 26 to 28 wk of age [
11]. The aged hens have a relatively fragile physique and are more susceptible to dietary factors, which may partially explain the inconsistent effect of lutein on egg production between the present study and literature; however, this needs to be studied further. Additionally, egg mass, egg weight, and feed intake with less importance than egg number for aged breeder hens were not shown and discussed in the present study.
In avian species, egg production is mainly presupposed by follicular grades. Due to the substantial size of chicken follicles, the average diameter can be used to compare follicular grades. Follicular development involves a complex process which ultimately results in ovulation. At the same time, thousands of developing follicles undergo atresia. Studies showed that antioxidants strongly benefit follicular development and their size increase [
12,
14,
17]. Carotenoids as one of the most important antioxidants in the nature were also shown to have effective antioxidation in the ovary. Literature reported that carotenoid lycopene decreased oxidative stress and improved the function of D-gal-induced and naturally aged ovaries [
18]. Lutein increased the numbers of normal oocytes and oocyte quality in a polycystic ovary syndrome model [
7]. Literature about dietary lutein’s effect on follicular development in poultry is scarce. In the present study, the average diameters of follicles were increased in lutein diets, compared to CON, indicating that lutein can improve follicle growth and maturation, and alleviate the ovarian degeneration of aged hens, but more studies are needed.
The antioxidative activity of lutein on follicular development and quality may also have a cascade effect on fertilization, embryonic development, and hatchability. Indeed, in the present study, dietary lutein improved the percentages of fertile eggs, embryos, and healthy chicks. Literature about this is minimal. Bandariyan et al [
7] reported that lutein significantly increased fertilization and blastocysts and decreased arrested embryos. Since the antioxidant and immunostimulatory roles of carotenoids are critical during the immediate post-hatch period, maternal dietary intake of carotenoids exhibited important ramifications for the viability of chicks in the first week of age [
19]. Data are scarce about the effect of carotenoids, including lutein, on the percentages of hatchability and healthy chicks. As known, there are two peak periods of death during the chicken embryo development, distributed at 2 to 4 and 19 to 21 d of the embryo. The first death peak is mainly related to the inner quality of eggs and the second is involved in external hatching conditions. How the maternal lutein incorporated in the egg affects the dynamic and peak death of chicken embryos deserves further study.
The reproductive behavior of hens is strictly regulated by reproductive hormones, such as E2, FSH, LH, and PROG. Certain levels of these hormones are necessary to maintain egg production. In theory, these hormones are naturally secreted and fluctuate with physiological phases in a reproductive cycle. Non-hormonal external factors, such as dietary manipulation and environmental changes, can only have a slight impact on these hormones. However, the improvement in egg production from dietary manipulation can make a sizable profit for large-scale intensive output, especially for commercially important broiler chicken or turkey breeders who genetically have poor reproduction [
12,
14]. In the present study, the relatively high levels of E2, FSH, LH, and PROG in lutein diets may partially explain the increased effect on egg production. Literature about the direct relationship between dietary lutein and reproductive hormones is scarce. Indirectly, lutein ameliorated reproductive damage by alleviating oxidative stress, inflammation, and apoptosis in male rats [
8]. Serum antioxidants were associated with serum reproductive hormones and ovulation among healthy women [
20]. Anyway, whether the lutein supplementation and reproductive release reflect a cause and effect relationship remains to be further determined.
Oral lutein enters the bloodstream, travels throughout the body, incorporates into some tissues, and influences the body’s health. Getting back to the fundamentals, all of the health benefits of lutein are the result of its antioxidative activity. Lutein presence at a particular concentration in the body prerequisites its antioxidation, and the effect, weak or strong, is putatively dependent on the concentration of lutein. Hens, by feeding lutein fortified
Chlorella, significantly increased lutein contents in the serum, liver, growing oocytes, and eggs [
21]. Similarly, lutein concentration varied from 0.04 to 0.68 μg/g in the eye, brain, heart, lung, intestine, pancreas, kidney, and breast of hens fed with a lutein-enriched diet [
22]. In the present study, hens in lutein treatments had higher levels of lutein in the serum and yolk, and lower concentrations of oxidative products, including MDA, PCO, and 8-OHdG, indicating that the oxidative damages of the body were decreased in lutein diets.
Oxidative damage in the body is widespread, mainly manifested as damage to the structure and function of biological macromolecules, which leads to genetic mutations, cell carcinogenesis and individual aging. The MDA, PCO, and 8-OHdG are the oxidative products of lipids, proteins, and deoxyribonucleic acids, respectively. Lutein intervention decreased serum MDA and 8-OHdG in rats [
23]. Carotenoid blends including lutein decreased MDA in broilers [
24]. Lutein treatment blocked the high glucose-mediated elevation of intracellular reactive oxygen species, PCO, and MDA content in ARPE-19 cells [
25]. Lutein protected against vancomycin-induced renal injury by decreasing MDA, PCO, and related inflammatory and apoptotic signal pathways [
26]. The decreases in the oxidative terminal products of biological macromolecules by lutein in the present study and the literature demonstrated the protective effect of lutein in the body. Interestingly, lutein derived from marigold petals suppressed cancer cells by triggering reactive oxygen species generation and activating apoptotic genes [
27]. The paradoxical responses of lutein to reactive oxygen species and cell survival in normal and abnormal cells needs further study.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





