 |
 |
| Anim Biosci > Volume 36(7); 2023 > Article |
|
Abstract
Objective
Present study was designed to evaluate the efficacy of Acacia nilotica bark extract as an alternative to antibiotic growth promoters in broilers.
Methods
Six hundred, day-old broiler chicks were randomly divided into six groups (NC, without any supplementation; AB, NC+Zinc Bacitracin; PB, NC+Safmannan; ANBE1, NC+A. nilotica bark extract 0.1%; ANBE3, NC+A. nilotica bark extract 0.3%; ANBE5, NC+A. nilotica bark extract 0.5%), with ten replicates per group (10 chicks/replicate) and feeding trial was lasted for 35 days.
Results
Results showed that weight gain (1,296.63 g) and feed conversion ratio (FCR, 1.59) of AB was better than NC, during the finisher phase. Overall FCR of AB (1.53), PB (1.54), and ANBE5 (1.54) was significantly (p<0.05) better than NC. From carcass parameters relative weight of wing and heart were highest in ANBE3 (2.5% and 1.51%, respectively). Significantly (p<0.05) highest blood glucose level was observed in NC (264.5 mg/dL) and highest albumin concentration was found in AB (1.46 mg/dL). In addition, antibody titer levels against ND and IBD were higher in ANBE5 than NC, while higher relative weight of bursa was observed in ANBE3 than NC. The villus height to crypt depth ratio in all experimental groups was better than NC.
Antibiotics have been used for decades for treatment of bacterial infections and to improve production efficiency in the poultry industry. Notwithstanding the benefits of antibiotics, developing bacterial resistance against frequently used antibiotics is a global concern. Improper use of antibiotics for long duration have led to antibacterial resistance and development of multidrug-resistant strains of bacteria [1], which could be transferred to other farm animals and humans [2]. Use of food contaminated with antibiotic resistant bacteria is a key point to consider because it might become challenging and costly to treat infection caused by such types of bacteria [3,4]. Several European countries have banned the use of antibiotics at sub-therapeutic levels [5]. In those countries there is a decrease in the growth performance of poultry birds and increase in rate of mortality due to bacterial infections such as salmonellosis, necrotic enteritis and colibacillosis [6]. Based on these premises, farmers, nutritionists, and pharmacists have been searching for suitable alternative which have no residual effect and maintain the growth performance of poultry birds at optimum levels [7].
Natural plant and herbal products could be novel antimi crobial agents with potentially new modes of action [8]. Active principles found in plants could be a safe and effective alternative to synthetically produced antimicrobial agents. The active principles found in different plants are volatile essential oils, alkaloids, phenols, resins, phenolic oleosins, glycosides, tannins, steroids, and terpenes which have antibacterial activities against different bacteria [9] and have antioxidant effect [10]. Among medicinally important plants Acacia nilotica has its own importance due to its many medicinal uses and antioxidant activity. Work has been done on the extract of different parts of A. nilotica which showed that it contains many phytogenic feed additives which have beneficial effects not only in poultry but also in humans [11]. A. nilotica has been reported to improve the growth performance in poultry [12]. A. nilotica could improve the health status of poultry birds and increase the bioactive compounds index in poultry meat [13]. It has been reported that extract of different medicinal plants improves the nutrients digestibility by enhancing the secretions of digestive enzymes which led to improved growth performance in poultry birds [14]. They also improved gut health by enhancing the permeability of cell membranes for nutrient absorption and killing the pathogenic bacteria [14,15]. Keeping in view the growing concern of antibiotic resistance and importance of A. nilotica in poultry, present study was planned to evaluate the efficacy of the use of bark extract of A. nilotica on different biological parameters in broilers and to compare it with antibiotics.
The protocol of this experiment was approved by committee of Ethical Handling of Experimental Birds, University of Veterinary and Animal Sciences, Lahore, Pakistan, for the care and use of experimental birds with approval number: DAS/358, Dated 03/05/2021.
Stem bark of mature and healthly A. nilotica was collected from Lahore and Kasur, Punjab, Pakistan and washed thoroughly under running water to remove dirt, followed by rinsing with sterilized distilled water. Bark was oven dried at low temperature and ground with an efficient grinding machine with a sieve of 2 mm. Ground bark powder (100 g) was extracted with absolute ether (600 mL) through Soxhlet Extraction technique [16]. Maximum extract was collected after 10 to 12 extraction cycles. Extract was concentrated by using rotary evaporator under high pressure (>60 Pascal) and controlled temperature (40┬░C to 50┬░C). The extract was then placed in oven at 40┬░C for four days for maximum removal of moisture. The extract then placed in plastic zip bags at ŌłÆ20┬░C till further analysis or use. Phytochemical analysis of extract was done for presence or absence of tannins, alkaloids, terpenoids, glycosides, saponins, steroids and flavonoids, following previous methods [16ŌĆō18]. Bark extraction and phytochemical analysis were performed in the Department of Animal Nutrition, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Six hundred, day-old broiler chicks (Cobb-500) were divided into six groups (NC, AB, PB, ANBE1, ANBE3, and ANBE5) consisted of ten replicates each (10 chicks per replicate). NC, negative control with no supplementation; AB, positive control, NC+Zinc Bacitracin; PB, probiotic supplemented group, NC+Safmannan; ANBE1, NC+A. nilotica bark extract 0.1%; ANBE3, NC+A. nilotica bark extract 0.3%; ANBE5, NC+A. nilotica bark extract 0.5%. A. nilotica bark extract was sprayed after pelleting on to the cooled pellets. Feeding trial was conducted at the R&D farm of Sultan Feed Pvt. Ltd., Sargodha, Pakistan, for 35 days, divided into two phases: starter phase 1 to 21 days; finisher phase 22 to 35 days. Diet of the negative control group was formulated according to ŌĆ£Cobb-500 Nutritional Requirement Manual Guide, 2018ŌĆØ (Table 1). Free access to feed and water was provided to the birds throughout the experiment.
During feeding trial feed intake and weight gain were recorded on weekly bases. Data were arranged to calculate average feed intake, weight gain and feed conversion ratio (FCR), during starter and finisher phases and on overall basis.
At the end of the experiment, 1 bird/replicate was selected and slaughtered to determine the effect of dietary treatments on carcass characteristics. Live body weight of selected birds was recorded and carcass weight was also recorded after slaughtering. Dressing percentage was calculated from data of live body weight and carcass weight. While relative weight of different parts (breast, thigh, and wing) and internal organs (heart, liver, and gizzard) were calculated as a percent of carcass weight.
Blood samples (about 3 mL form each bird) were collected from jugular vein at the time of slaughtering of birds and serum was separated by centrifugation, which was collected in Eppendorf tubes and stored at ŌłÆ15┬░C to ŌłÆ20┬░C until further analysis. Serum was analyzed for total protein, albumin, globulin, blood glucose and triglyceride levels using respective kits [19].
Immune response was analyzed by determining the levels of haemagglutination inhibition titer against Newcastle disease virus (NDV) and ELISA titer level against infectious bursal disease virus (IBDV) [20]. In addition, immune organ indexes were recorded.
Jejunum was separated from slaughtered birds and about 2 cm part was cut and rinsed with normal saline and stored in 10% neutral formalin solution. Preserved samples was used to prepare slides by sectioning it using a microtome (5 ╬╝m). Hematoxylin and eosin staining was done followed by morphometric analysis using a microscope having a computer assisted morphometric system (Nikon Corporation, Tokyo, Japan).
Data collected for all parameters were arranged and expressed as mean┬▒standard error of the mean. All data were analyzed through one-way analysis of variance using SPSS 26. TukeyŌĆÖs test was used to determine statistical difference among the treatments at significance level of p<0.05.
Screening of bark extract of A. nilotica revealed that it contains tannins, alkaloids, terpenoids, glycosides and saponins. On the other hand, steroids and flavonoids were not detected in the extract (Table 2).
No significant effect of dietary treatments was observed on all parameters of growth performance during starter phase. During finisher phase weight gain and FCR in AB was significantly (p<0.05) better than NC but similar to A. nilotica bark extract supplemented groups. Similar effect was observed on weight gain during overall experiment. However, FCR of AB, PB, and ANBE5 was significantly better than NC on overall bases (Table 3).
Dressing percentage varied from 68.29% to 69.43% with highest in PB and lowest in NC. Numerically highest relative breast weight was observed in AB (23.50%) followed by PB (23.26%) and lowest in ANBE5 (22.22%). Highest (p<0.05) relative weight of wing (2.5%) was observed in ANBE3 which was statistically similar to other groups, except ANBE5. Higher (p<0.05) relative weight of heart (1.51%) was found in ANBE3 than NC, AB, and PB groups (Table 4).
Effect of dietary treatments was also evaluated on different parameters of blood biochemistry and the result exhibited no significant difference among the groups except glucose and albumin levels (Table 5). Significantly (p<0.05) highest blood glucose level was observed in NC (264.5 mg/dL) and lowest in ANBE5 (213.75 mg/dL). Total protein levels were not affected; however, highest albumin levels were found in AB (1.46 mg/dL) followed by NC (1.36 mg/dL) and lowest was observed in ANBE5 (1.20 mg/dL). Levels of triglyceride in blood serum varied from 48.57 to 61.25 mg/dL in ANBE1 and NC, respectively.
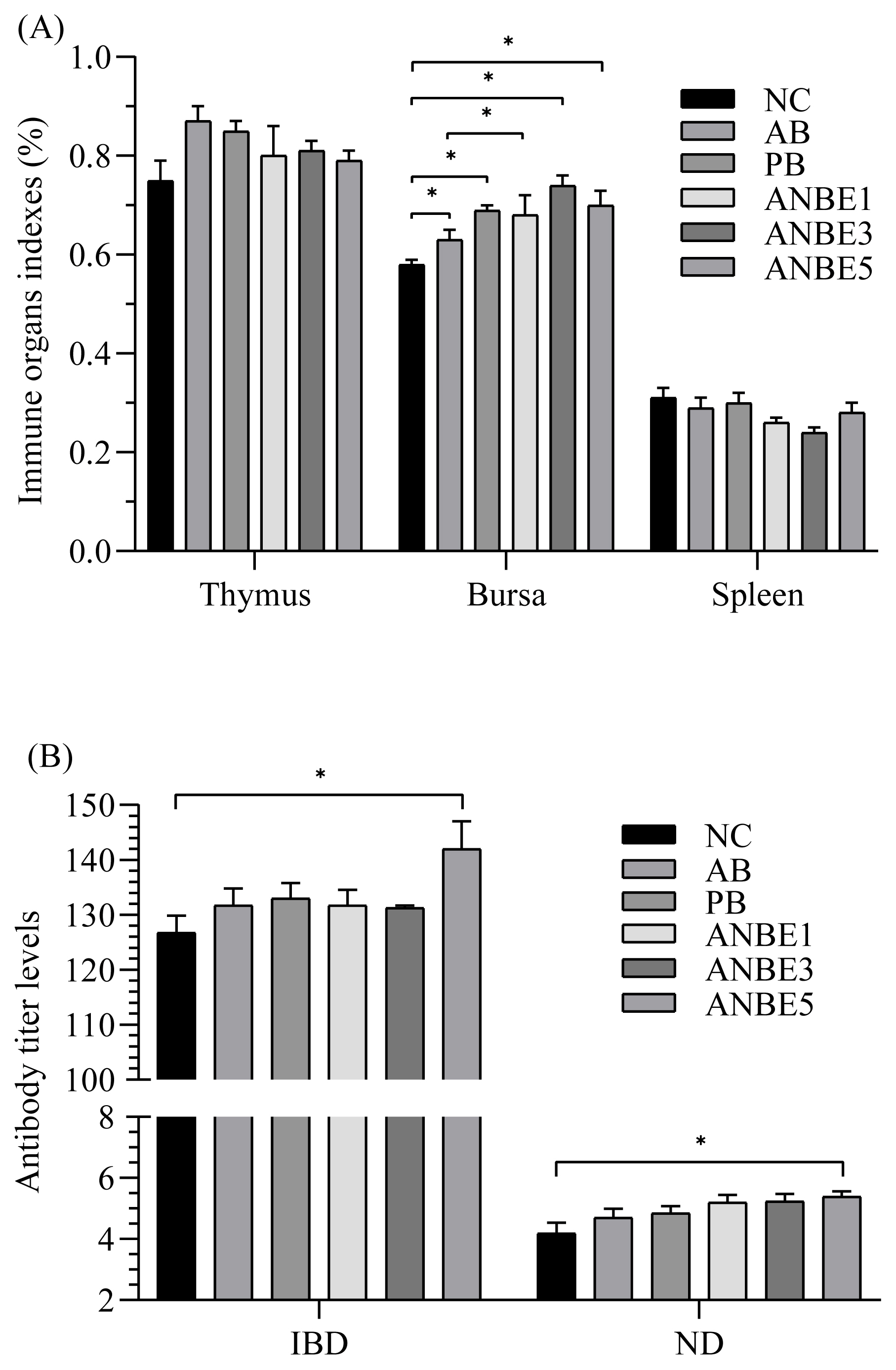
Immune response was analyzed by evaluating the effect of dietary treatments on relative weight of immune organs (Figure 1A) and antibody titer levels against NDV and IBDV (Figure 1B). Significantly (p<0.05) higher antibody titer levels against NDV and IBDV were observed in ANBE5 (5.38; 142) than NC (4.18; 126.75), however it was statistically similar to other groups. Relative weight of bursa in ANBE3 (0.74%) was statistically similar to other experimental groups (0.68% to 0.70%) and PB (0.69%) but significantly (p<0.05) higher than NC (0.58%). Relative weight of spleen and thymus was statistically similar among the different groups.
No significant effect of dietary treatments was found on villus height but crypt depth and villus height to crypt depth ratio were affected (Table 6). Villus height varied from 1,201.60 ╬╝m to 1,305.72 ╬╝m, with lowest in NC and highest in ANBE5. Highest crypt depth was found in NC (234.83 ╬╝m) and lowest in ANBE5 (204.93 ╬╝m). The villus height to crypt depth ratio in all experimental groups (5.95 to 6.37) and AB (6.34) groups was similar but statistically (p<0.05) higher than NC (5.13).
Extract of different parts of A. nilotica have been used in traditional medicines in different countries including Chinese, Unani, Ayurvedic and Egyptian for centuries because of the presence of different bioactive secondary compounds. Extracts of different parts of A. nilotica have bioactive compounds which support the health status in animals and humans and used for prevention and treatment of different diseases. Tannins, saponins, and flavonoids are used in digestive disorders; saponins and triterpenoids in cancer and polyphenols as antioxidants [21]. Screening analysis of bark extract confirmed the presence of tannins, alkaloids, terpenoids, glycosides and saponins, while steroids and flavonoids were absent. Results showed that overall weight gain and FCR were improved with A. nilotica bark extract supplementation in broilers, while carcass characteristics were not affected. Results of growth performance suggested that boilers could grow better in the absence of antibiotics with supplementation of A. nilotica bark extract and these results are in line with Abudabos et al [22], who stated that A. nilotica extract could effectively support the growth performance in broilers similar to antibiotics. Present results agree with that of Festus et al [23], who stated that herbal extract of A. nilotica, Moringa oleifera and wild mushroom could be used as a replacement for AGPs without affecting growth performance and carcass parameters in broilers. Similarly, Ngambi et al [12] stated that supplementation of leaf extract of A. nilotica had no significant effect on feed intake in broilers however fat weight was reduced. Present results are in line with previous study of Marimuthu and DŌĆÖSouza [24], who stated that commercial choline chloride could be replaced with herbal mixture of Curcuma longa and A. nilotica in broilers. Herbal mixture supported the growth-related parameters as done by choline chloride. In another study, inclusion of 10% A. nilotica seed meal significantly improved weight gain and FCR with lowest feed cost per unit weight gain in broilers [25]. A. nilotica leaf meal supplementation also increase the weight gain and economic efficiency with suitable FCR in broilers [26]. Improved growth performance and FCR during this experiment and previous studies [13ŌĆō15] might be due to presence of bioactive compounds in extract which stimulate the production of digestive enzymes [15] and improved the nutrientsŌĆÖ digestibility [27,28].
Analysis of blood biochemistry showed that A. nilotica extract reduced the blood glucose levels and enhanced albumin levels in broilers, while other components remained unaffected. Similarly, albumin levels increased in boilers with supplementation of phytogenic additives in broilers [15]. Previous study confirmed that A. nilotica extract controlled blood sugar level in hyperglycemic rats and maintained normal blood lipid profile [29]. Another study stated that intraperitoneal injection of A. nilotica bark extract is an effective way to control blood sugar level in diabetes model induced in mice [30]. Additional study conducted on rabbits, in which diabetes was induced by alloxan, suggested that A. nilotica pods extract significantly reduced the blood glucose level and maintained the blood lipids profile [31]. Olorede et al [25] stated that use of A. nilotica seed meal at the levels of 20% significantly increased hemoglobin, packed cell volume and red blood cells without compromising the body weight in broilers.
Immune response was analyzed by evaluating the effect of dietary treatments on immune organs indexes and antibody titer levels against ND and IBD. Results suggested that A. nilotica extract has some role in stimulation of immune system, as antibody titer levels were enhanced by A. nilotica extract supplementation and relative weight of bursa was also improved and results are supported by previous results that A. nilotica pod extract stimulated the immune system in rats [32]. A. nilotica pod extract supplementation enhanced the counts of white blood cells [32], which are the primary line of defense against infection and tissue damages [33], which suggested that bioactive compounds of extract stimulate the immune system to protect the body of the host animal. Another study stated that A. nilotica extract boosted the proliferation of splenocytes [34] and these findings were also supported by in vitro analysis [35]. Enhanced proliferation of splenocytes is the indication of activation of cellular immune response in animals [34] which are suppressed by immune suppression activities of pathogenic microorganisms [36].
Gut health has an important role maintaining the health status as it is the main site of digestion and absorption of nutrients. Intestinal morphological parameters (villus height, crypt depth, and their ratio) are indicators of gut health, its maturity and functionality [37] which are mainly affected by dietary manipulation [38]. Improved morphological parameters suggested better gut health required to improve the feed efficiency and support the health of animals. In present study intestinal morphology was improved by A. nilotica bark extract supplementation, which might be the reason for improved FCR and better weight gain in broilers. Similar to AGPs, A. nilotica extract could induce modification in immune system and microbial load at intestinal levels. Decrease in microbial load and reduction in unnecessary use of energy lower the energy wastage and enhance growth performance. In addition, strong immune system with reduced load of pathogenic microbes reduces the incidence of diseases in broilers [39].
Notes
ACKNOWLEDGMENTS
The authors acknowledged the Sultan Feed Pvt. Ltd., Sargodha, Pakistan for providing necessary facilities to conduct feeding trial.
Figure┬Ā1
Effect of Acacia nilotica bark extract on immune response in broiler. (A) Relative weight of immune organs; (B) antibody titer levels. NC, negative control; AB, antibiotic supplemented group; PB, probiotic supplemented group; ANBE1, ANBE3, and ANBE5 were supplemented with A. nilotica bark extract at the levels of 0.1%, 0.3%, and 0.5%, respectively. Statistic difference from corresponding control value for a given parameter is annotated by: * p<0.05.
Table┬Ā1
Composition of negative control diets
| Items | Starter diet | Grower diet |
|---|---|---|
| Ingredients composition (%) | ||
| ŌĆāMaize | 55.61 | 59.13 |
| ŌĆāSoybean meal | 25 | 20 |
| ŌĆāGuar meal | 3 | 3 |
| ŌĆāCanola meal | 10 | 10 |
| ŌĆāCorn gluten 60% | 0.11 | 1.89 |
| ŌĆāVeg. oil | 3 | 3.84 |
| ŌĆāLysine sulphate | 0.5 | 0.46 |
| ŌĆāMethionine-DLM | 0.27 | 0.022 |
| ŌĆāL-Theronine | 0.01 | 0.07 |
| ŌĆāDicalcium phosphate | 1 | 0.89 |
| ŌĆāCalcium carbonate | 0.7 | 0.69 |
| ŌĆāSodium bicarbonate | 0.07 | 0.12 |
| ŌĆāSodium chloride | 0.24 | 0.2 |
| ŌĆāPremix1) | 0.5 | 0.5 |
| ŌĆāTotal | 100 | 100 |
| Nutrients composition (%) | ||
| ŌĆāME (Kcal/kg) | 3,000 | 3,150 |
| ŌĆāCrude protein | 21 | 19.5 |
| ŌĆāDig. Lysine | 1.18 | 1.05 |
| ŌĆāDig. methionine | 0.45 | 0.42 |
| ŌĆāCalcium | 0.9 | 0.84 |
| ŌĆāAvailable phosphorus | 0.45 | 0.42 |
1) Vit. A 15,000 IU/kg, B1 2 mg/kg, B2 8 mg/kg, B6 4 mg/kg, B12 1 mg/kg, D3 3,000 IU/kg, E 60 IU/kg, K3 3 mg/kg, pantothenic acid 15 mg/kg, niacin 45 mg/kg, folic acid 1 mg/kg, choline chloride (60%) 500 mg/kg, manganese sulphate 18.5 mg/kg, magnesium sulphate 53 mg/kg, zinc sulphate 2 mg/kg, copper sulphate 45 mg/kg and ferrous sulphate 35 mg/kg.
Table┬Ā2
Phytochemicals found in Acacia nilotica bark extract
| Compounds | Availability |
|---|---|
| Tannins | Present |
| Terpenoids | Present |
| Alkaloids | Present |
| Glycosides | Present |
| Saponins | Present |
| Steroids | Absent |
| Flavonoids | Absent |
Table┬Ā3
Effect of Acacia nilotica bark extract on growth performance in broiler
| Parameters | Treatments1) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| NC | AB | PB | ANBE1 | ANBE3 | ANBE5 | |||
| Starter phase | ||||||||
| ŌĆāFeed intake (g) | 1,432.81 | 1,427.08 | 1,390.68 | 1,452.43 | 1,411.45 | 1,418.16 | 8.52 | 0.583 |
| ŌĆāWeight gain (g) | 984.65 | 991.03 | 970.89 | 999.46 | 986.22 | 991.11 | 3.89 | 0.870 |
| ŌĆāFCR | 1.46 | 1.44 | 1.43 | 1.46 | 1.43 | 1.43 | 0.00 | 0.988 |
| Finisher phase | ||||||||
| ŌĆāFeed intake (g) | 2,057.63 | 2,063.14 | 2054.59 | 2,017.36 | 2,042.98 | 2,038.82 | 6.79 | 0.917 |
| ŌĆāWeight gain (g) | 1,140.58b | 1,296.63a | 1268.21ab | 1,149.64ab | 1,214.33ab | 1,261.43ab | 26.57 | 0.017 |
| ŌĆāFCR | 1.81b | 1.59a | 1.62ab | 1.77ab | 1.68ab | 1.62ab | 0.04 | 0.019 |
| Overall | ||||||||
| ŌĆāFeed intake (g) | 3,490.44 | 3,490.22 | 3,445.27 | 3,469.79 | 3,454.43 | 3,456.97 | 7.79 | 0.957 |
| ŌĆāWeight gain (g) | 2,125.23b | 2,287.67a | 2,239.09ab | 2,149.10ab | 2,200.54ab | 2,252.55ab | 25.60 | 0.025 |
| ŌĆāFCR | 1.64b | 1.53a | 1.54a | 1.62b | 1.57ab | 1.54a | 0.02 | 0.001 |
Table┬Ā4
Effect of Acacia nilotica bark extract on carcass characteristics in broiler
| Parameters (%) | Treatments1) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| NC | AB | PB | ANBE1 | ANBE3 | ANBE5 | |||
| Dressing percentage | 68.29 | 69.21 | 69.43 | 68.33 | 68.40 | 68.40 | 0.23 | 0.080 |
| As a percent of carcass weight | ||||||||
| ŌĆāBreast | 22.35 | 23.50 | 23.26 | 21.64 | 21.53 | 22.22 | 0.33 | 0.177 |
| ŌĆāThigh | 19.58 | 19.26 | 19.04 | 19.56 | 19.26 | 19.22 | 0.09 | 0.715 |
| ŌĆāWing | 2.16ab | 2.20ab | 2.35ab | 2.18ab | 2.50a | 1.89b | 0.08 | 0.052 |
| ŌĆāHeart | 1.21c | 1.26bc | 1.22bc | 1.32abc | 1.51a | 1.45ab | 0.05 | 0.003 |
| ŌĆāLiver | 3.66 | 3.86 | 3.71 | 3.78 | 3.86 | 3.86 | 0.04 | 0.885 |
| ŌĆāGizzard | 3.85 | 3.81 | 3.96 | 3.62 | 3.92 | 4.17 | 0.07 | 0.101 |
Table┬Ā5
Effect of Acacia nilotica leave extract on blood biochemistry in broiler
| Parameters (mg/dL) | Treatments1) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| NC | AB | PB | ANBE1 | ANBE3 | ANBE5 | |||
| Glucose | 264.50a | 229.50ab | 224.75b | 240.00ab | 244.25ab | 213.75b | 7.21 | 0.006 |
| Total protein | 2.58 | 2.62 | 2.63 | 2.61 | 2.48 | 2.36 | 0.04 | 0.295 |
| Albumin | 1.36ab | 1.46a | 1.33ab | 1.31ab | 1.33ab | 1.20b | 0.03 | 0.049 |
| Globulin | 1.22 | 1.16 | 1.30 | 1.30 | 1.15 | 1.17 | 0.03 | 0.874 |
| Triglyceride | 61.25 | 55.25 | 50.75 | 48.75 | 49.75 | 55.75 | 1.94 | 0.357 |
Table┬Ā6
Effect of Acacia nilotica bark extract on intestinal morphology in broiler
| Parameters | Treatments1) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| NC | AB | PB | ANBE1 | ANBE3 | ANBE5 | |||
| Villus height (VH, ╬╝m) | 1,201.60 | 1,300.75 | 1,238.01 | 1,244.32 | 1,263.98 | 1,305.72 | 16.23 | 0.091 |
| Crypt depth (CD, ╬╝m) | 234.83a | 205.79b | 219.42ab | 209.16ab | 211.03ab | 204.93b | 4.64 | 0.031 |
| VH/CD | 5.13b | 6.34a | 5.67ab | 5.95a | 6.00a | 6.37a | 0.19 | 0.001 |
REFERENCES
1. Yaqoob MU, Wang G, Wang M. An updated review on probiotics as an alternative of antibiotics in poultry. Anim Biosci 2022; 35:1109ŌĆō20. https://doi.org/10.5713/ab.21.0485



2. Sorum H, Sunde M. Resistance to antibiotics in the normal flora of animals. Vet Res 2001; 32:227ŌĆō41. https://doi.org/10.1051/vetres:2001121


3. Mathew AG, Cissell R, Liamthong S. Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. Foodborne Path Dis 2007; 4:115ŌĆō33. https://doi.org/10.1089/fpd.2006.0066


4. Akbar A, Anal AK. Isolation of Salmonella from ready-to-eat poultry meat and evaluation of its survival at low temperature, microwaving and simulated gastric fluids. J Food Sci Technol 2015; 52:3051ŌĆō7. https://doi.org/10.1007/s13197-014-1354-2


5. Castanon JIR. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci 2007; 86:2466ŌĆō71. https://doi.org/10.3382/ps.2007-00249


6. Diarra MS, Malouin F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front Microbiol 2014; 5:282https://doi.org/10.3389/fmicb.2014.00282



7. Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 2011; 24:718ŌĆō33. https://doi.org/10.1128/CMR.00002-11



8. Braga LC, Leite AAM, Xavier KGS, et al. Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus. Can J Microbiol 2005; 51:541ŌĆō7. https://doi.org/10.1139/w05-022


9. Mitcher LA, Okwute SK, Gollapudie SR, Drake S, Anova E. Antimicrobial pterocarpans of nigerian Erythrina mildbraedii. Phytochemistry 1988; 27:3449ŌĆō52. https://doi.org/10.1016/0031-9422(88)80746-5

10. Akinmoladun AC, Ibukun EO, Afor E, et al. Chemical constituents and antioxidant activity of Alstonia boonei. Afr J Biotechnol 2007; 6:1197ŌĆō201.
11. Surai PF. Selenium in poultry nutrition. Reproduction, egg and meat quality and practical applications. WorldŌĆÖs Poult Sci J 2002; 58:431ŌĆō50. https://doi.org/10.1079/WPS20020032

12. Ngambi JW, Nakalebe PM, Norris D, Malatje MS, Mbajiorgu CA. Effects of dietary energy level and tanniferous Acacia karroo leaf meal level of supplementation at finisher stage on performance and carcass characteristics of Ross 308 broiler chickens in South Africa. Int J Poult Sci 2009; 8:40ŌĆō6.

13. Lee K, Kim J, Oh S, Kang C, An B. Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J Poult Sci 2015; 52:15ŌĆō22. https://doi.org/10.2141/jpsa.0140073

14. Kim S, Lee K, Kang C, An B. Growth performance, relative meat and organ weights, cecal microflora, and blood characteristics in broiler chickens fed diets containing different nutrient density with or without essential oils. Asian-Australas J Anim Sci 2016; 29:549ŌĆō54. https://doi.org/10.5713/ajas.15.0426



15. Amad AA, Wendler KR, Zentek J. Effects of a phytogenic feed additive on growth performance selected blood criteria and jejunal morphology in broiler chickens. Emir J Food Agric 2013; 25:549ŌĆō54. https://doi.org/10.9755/ejfa.v25i7.12364

16. Odebiyi OO, Sofowora EA. Phytochemical screening of Nigerian medicinal plants II. Lloydia 1978; 41:234ŌĆō46.

17. Gundidza M. Phytochemical screening of some Zimbabwean medicinal plants. Cent Afr J Med 1985; 31:238ŌĆō9. https://doi.org/10.10520/AJA00089176_1836


18. Ebana RUB, Madunagu BG, Etok CA. Antimicrobial effect of Strophanthus Lipidis and Seramone afzelii on some pathogen bacterial and their drug resistant strains. Nig J Bot 1993; 6:27ŌĆō31.
19. Kumar B, Kumbhakar NK. Haematobiochemical profile of Aseel in Chhattisgarh region. Indian Vet J 2015; 92:40ŌĆō2.
20. Xie D, Wang ZX, Dong YL, et al. Effects of monochromatic light on immune response of broilers. Poult Sci 2008; 87:1535ŌĆō9. https://doi.org/10.3382/ps.2007-00317


21. Saini ML, Saini R, Roy S, Kumar A. Comparative pharmacognostical and antimicrobial studies of acacia species (Mimosaceae). J Med Plant Res 2008; 2:378ŌĆō86. https://doi.org/10.5897/JMPR.9000378

22. Abudabos AM, Abdullah HA, Yousif MD, Khan RU. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environ Sci Pollut Res 2016; 23:24151ŌĆō7. https://doi.org/10.1007/s11356-016-7665-2

23. Festus N, Bright SG, Orile JE, Anita F. Effect of oral administration of aqueous extract of Moringa oleifera seeds, gum arabic and wild mushroom on growth performance of broiler chickens. Inter J Med Plant Res 2016; 5:200ŌĆō8.
24. Marimuthu S, DŌĆÖSouza P. Field performance of Kolin Plus, a poly herbal formulation to replace synthetic choline chloride. Int J Vet Sci Anim Husb 2019; 4:32ŌĆō4.
25. Olorede BR, Alade AR, Ajagbonna OP. Effects of substituting groundnut cake with acacia seed kernel meal on performance, haematology, serum biochemical parameters and economy of production of broilers. Trop J Anim Sci 2000; 3:107ŌĆō15.

26. El-Galil A, Hassan MM, Abu El-Soud KM, El-Dayem A, Salem FM. Utilization of acacia saligna leaf meal as a non-traditional feedstuff by local growing hens under desert conditions. Egypt J Nutr Feeds 2019; 22:211ŌĆō7.

27. Buchanan NP, Hott JM, Cutlip SE, Rack AL, Asamer A, Moritz JS. The effects of a natural antibiotic alternative and a natural growth promoter feed additive on broiler performance and carcass quality. J Appl Poult Res 2008; 17:202ŌĆō10. https://doi.org/10.3382/japr.2007-00038

28. Khan RU, Naz S, Nikousefat Z, Tufarelli V, Laudadio V. Thymus vlugaris: alternative to antibiotics in poultry feed. WorldŌĆÖs Poult Sci J 2012; 68:401ŌĆō8. https://doi.org/10.1017/S0043933912000517

29. Natarajan M, Srinivasan M. Antidiabetic and antioxidant activity of Acacia nilotica leaf on alloxan induced diabetic rats. Int J Pharm Biol Sci 2015; 6:110ŌĆō26.
30. Mukundi MJ, Piero NM, Mwaniki NE, et al. Antidiabetic effects of aqueous leaf extracts of acacia nilotica in alloxan induced diabetic mice. J Diabetes Metab 2015; 6:1000568https://doi.org/10.4172/2155-6156.1000568

31. Abuelgassim AO. Antioxidant potential of date palm leaves and Acacia nilotica fruit in comparison with other four common Arabian medicinal plants. Life Sci J 2013; 10:3405ŌĆō10.
32. Umaru B, Saka S, Mahre MB, Dogo HM, Ojo NA, Onyiyili PA. Effects of aqueous pod extract of Acacia nilotica on white blood cells, platelets and clotting time in albino rats. Am J Pharmacol Pharmacother 2016; 3:001ŌĆō006. https://doi.org/10.21767/2393-8862.100035

33. Ladokun O, Ojezele M, Arojojoye O. Comparative study on the effects of aqueous extracts of viscum album (mistletoe) from three host plants on hematological parameters in albino rats. Afr Health Sci 2015; 15:606ŌĆō12. https://doi.org/10.4314/ahs.v15i2.38



34. Singh S, Taneja M, Majumdar DK. Biological activities of Ocimum sanctum L. fixed oil-an overview. Indian J Exp Biol 2007; 45:403ŌĆō12.

35. Koko WS, Mesaik MA, Yousaf S, Galal M, Choudhary MI. In vitro immunomodulating properties of selected Sudanese medicinal plants. J Ethnopharmacol 2008; 118:26ŌĆō34. https://doi.org/10.1016/j.jep.2008.03.007


36. Sharma AK, Amit K, Sharad KY, Rahal A. Studies on antimicrobial and immunomodulatory effects of hot aqueous extract of acacia nilotica L. leaves against common veterinary pathogens. Vet Med Int 2014; 2014:747042https://doi.org/10.1155/2014/747042



37. Moghaddam HN, Salari S, Arshami J, Golian A, Maleki M. Evaluation of the nutritional value of sunflower meal and its effect on performance, digestive enzyme activity, organ weight, and histological alterations of the intestinal villi of broiler chickens. J Appl Poult Res 2012; 21:293ŌĆō304. https://doi.org/10.3382/japr.2011-00396

38. Salim HM, Kang HK, Akter N, et al. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult Sci 2013; 92:2084ŌĆō90. https://doi.org/10.3382/ps.2012-02947


39. Vondruskova H, Slamova RB, Trckova M, Zraly ZB, Pavlik I. Alternatives to antibiotic growth promoters in prevention of diarrhoea in weaned piglets: a review. Vet Med 2010; 55:199ŌĆō224. https://doi.org/10.17221/2998-VETMED







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





