 |
 |
| Anim Biosci > Volume 37(4); 2024 > Article |
|
Abstract
Objective
Abnormally increased somatic cell counts (SCCs) in milk is usually a sign of bovine subclinical mastitis. Mutual interaction between the host and its associated microbiota plays an important role in developing such diseases. The main objective of this study was to explore the difference between cows with elevated SCCs and healthy cattle from the perspective of host-microbe interplay.
Methods
A total of 31 milk samples and 23 bovine peripheral blood samples were collected from Holstein dairy cattle to conduct an integrated analysis of transcriptomic and metagenomics.
Results
The results showed that Ralstonia and Sphingomonas were enriched in cows with subclinical mastitis. The relative abundance of the two bacteria was positively correlated with the expression level of bovine transcobalamin 1 and uridine phosphorylase 1 encoding gene. Moreover, functional analysis revealed a distinct alternation in some important microbial biological processes.
Conclusion
These results reveal the relative abundance of Ralstonia and Sphingomonas other than common mastitis-causing pathogens varied from healthy cows to those with subclinical mastitis and might be associated with elevated SCCs. Potential association was observed between bovine milk microbiota composition and the transcriptional pattern of some genes, thus providing new insights to understand homeostasis of bovine udder.
Bovine subclinical mastitis, characterized by elevated milk somatic cell counts (SCC), is not only associated with dairy animalsŌĆÖ health and welfare, but also with the quality of dairy products. Amongst numerous factors, bacterial infection is one of the major causes of subclinical mastitis. Under normal circumstances, the composition of microbial community is in dynamic balance in the mammary gland of lactating cows, however, various endogenous and exogenous upsets can disrupt intramammary homeostasis, resulting in dysbiosis in the mammary gland, thus, leads to mastitis or subclinical mastitis. The host-associated factors and microbial profile are main determinants of the bovine udder homeostasis [1,2].
Recently, multiple advances have been gained in studies of milk microbiota and its association with the bovine mammary health status. Historically, bacteria detected in milk was linked to external contamination but latest researches suggested otherwise [3]. Isolation and cultivation of milk bacteria has yielded a lot of results in common clinical or subclinical mastitis pathogens identification. Various metagenomics studies have shown that milk microbiota composition can reflect the mammary gland health state to greater extent. A shift in microbiota composition is reported in milk from subclinical mastitis cows [1,4ŌĆō6], however, there lacks a consensus on which microbiota constitutes the microbial community of milk from healthy quarters.
Host-microbe interplay, shaped by host defense against potential harmful microorganisms and the response of microorganisms to the host immune system attack, is present in many immune-mediated diseases including bovine mastitis and subclinical mastitis [2]. Alternation in host gene expression pattern of specific pathogen-induced subclinical mastitis has also been well studied in recent years [7]. Despite lots of bacterial infection experiments performed in vitro to elucidate host-bacteria interaction in bovine mammary diseases, the mechanism of the interplay between host and colonized microbiota as a whole remains vague. To our knowledge, a combined analysis of milk microbiota and host gene expression in attempt to unveil the relationship among host immune response, microbiota and bovine subclinical mastitis has not been reported till date.
The present study offers a new perspective to understand how microbes interact with the host in bovine subclinical mastitis and to elucidate the role of the gene-bacteria interplay in developing such diseases. We implemented a comparative analysis based on the host transcriptome and metagenome sequencing and investigated a differential gene expression profile between subclinical mastitis and healthy cows.
Procedures for collection of samples were approved by the Animal Welfare Committee of China Agricultural University, Beijing, China and were conducted strictly following the regulations and guidelines established by this committee (approval number: AW10111202-1-3). All cows sampled in this research were guaranteed to have no records of clinical mastitis for three consecutive months, and were reared in the same dairy farm in Beijing, Northern China. Milk samples collection was performed after a regular disinfection procedure i.e. teat cleaning with 70% ethanol and the first three streams of milk were discarded [8]. Milk from each of the four quarters was collected in a 50 mL-sterile falcon tube, evenly mixed together and kept on dry ice during the whole collection process. For each cow, two milk samples were taken with one put in ŌłÆ80┬░C for later DNA extraction and the other sent to Beijing Dairy Cattle Center for SCC measurement. Other phenotype information was acquired according to records provided by the dairy farm. A total of 23 peripheral blood samples were aseptically taken from caudal vein and stored in 10-mL pre-added ethylenediaminetetra-acetic acid-K2 vacutainers on the same day the milk samples were collected.
Total genomic DNA was extracted from milk using cetyltrimethyl ammonium bromide. The integrity and purity of DNA was tested using 1% agarose gel electrophoresis. Total RNA was isolated from peripheral white blood cells using Trizol reagent (Ambion, Waltham, MA, USA) following the manufacturerŌĆÖs instructions. Purity of RNA was measured by Nanodrop (Thermo Fish, Waltham, MA, USA) and RNA integrity was assessed using electrophoresis and Aglient 2100 BioAnalyzer. Qubit 2.0 Fluorimeter was used for accurate quantification of DNA and RNA. Both metagenomic shotgun sequencing and RNA-seq were performed on Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA), preceded by a construction of 150-bp paired-end library. The average percent of bases scoring Q30 or above were over 90% for reads generated from either metagenomic shotgun sequencing or RNA-seq techniques.
Based on a threshold of 100,000 SCC/mL, the samples were divided into MS (medium SCC) and LS (low SCC) group [8]. Low quality reads, adaptors and host reads filtering were accomplished using KneadData. The quality of all clean data was assessed utilizing FastQC first before subsequent analyses. Non-host reads that passed quality control were taken for taxonomic classification using Kraken2 and abundance re-estimation using Bracken (v 2.6). Eukaryotes and viruses were discarded from the analysis. De novo assembly of reads into contigs was conducted by Megahit (v 1.2.8) and protein-coding gene prediction was performed using Prodigal (v 2.6.3). Redundant sequences of coding sequence predicted were then removed by CD-HIT (v 4.8.1) in order to facilitate downstream analysis. The remaining non-redundant representative sequences then served as an index for Salmon alignment-free quantification and also the input to eggnog-mapper (v 2.0.1) for functional annotation. Alpha diversity indices (Shannon, Chao1, Simpson) at different sampling depths were calculated using function diversity using R package vegan. Difference of alpha diversity between the two groups was examined using Wilcoxon rank-sum test. Bray-Curtis distance matrices based on relative abundance table at different taxonomic levels were built up using function vegdist in vegan and permutational multivariate analysis of variance was done by adonis to examine the effect of various experimental factors (SCC, parity, lactation stage) on differences among samples (Bray-Curtis distance ~ SCC + parity + lactation stage). Dissimilarity among samples (beta-diversity) was visualized with function pcoa in ape package. To eliminate the effect of library size, edgeR was utilized to determine differentially abundant taxa (DAT) and metagenomic function.
Raw reads generated from RNA-seq were subjected to strict quality control (Trimmomatic v0.39) and were then aligned to Bos taurus ARS-UCD1.2 using STAR (v 2.7.9a) with an average mapping rate over 90%. Reads mapped to the reference genome were quantified by feature Counts in subread (v 2.0.2) package followed by TPM normalization using StringTie (v 2.1.5). Subsequent analyses were carried out using R package. Differentially expressed genes (DEG) were identified by edgeR. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis were conducted using online website KOBAS (kobas.cbi.pku.edu.cn/kobas3).
To find the correlation between co-expression gene modules and DAT, weighted correlation network analysis and module-trait relationship assessment were implemented by WGCNA using top 5,000 genes with the largest median absolute deviation (MAD). Pearson correlation coefficients of the relative abundance of DAT and genes both identified as DEGs and found in modules associated with DAT were calculated using function cor.test in R.
A total of 31 (MS = 13, LS = 18) milk samples were obtained and used for sole metagenomics analysis. The parity of each selected cow was less than three and days in lactation ranged from 54 to 164 d (97┬▒30.5 d). Distribution of SCC, parity and lactation are presented in Supplementary Figure S1. In general, 23 (MS = 8, LS = 15) samples that had both transcriptome data and metagenome data were contained in integrative analysis.
A total of 35 phyla were observed, of which 4 phyla (Proteobacteria 54.73┬▒14.35; Actinobacteria 16.47┬▒6.56; Firmicutes 19.71┬▒12.83; and Bacteroidetes 9.40┬▒6.70) were shared across all samples. Top 6 most abundant phyla selected based on the average relative abundance were Proteobacteria, Actinobacteria, Firmicutes, Bacteroidetes, Tenericutes, and Spirochaetes (Figure 1). Additionally, 70 classes, 166 orders, 375 families and 1,413 genera were identified. Species level were excluded from subsequent analyses due to inadequate sequencing depth for accurate classification at this level.
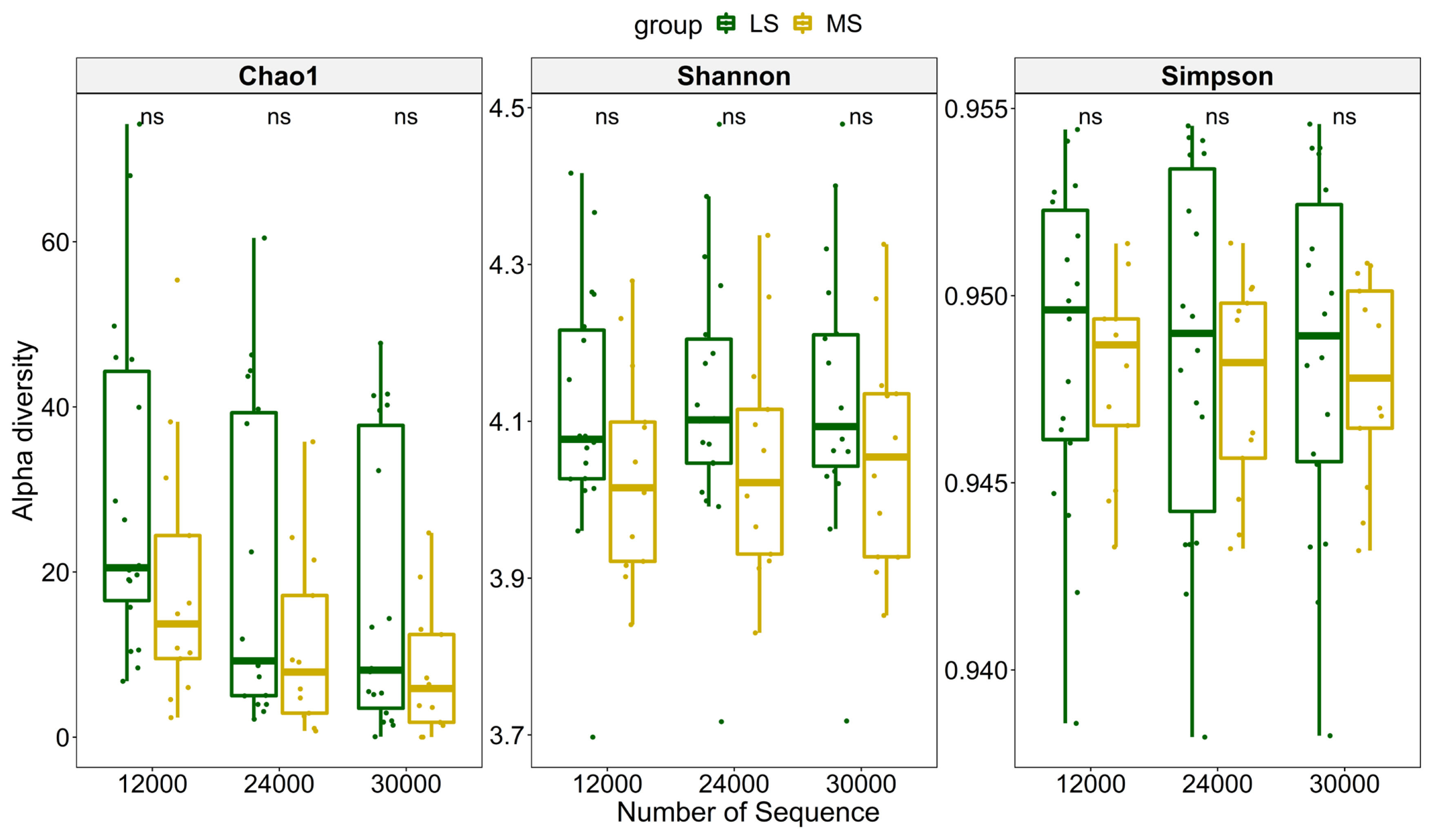
To compare richness of the microbial community in the two groups, three different indices were used to measure the overall diversity in each sample (Figure 2). The significance was calculated using paired Wilcoxon rank-sum test. The results showed that samples, subsampled at a depth of 12,000, 24,000, and 30,000 had no significant differences between MS and LS group using any of the three alpha diversity indices, indicating the richness difference was quite subtle. We further explored the impact of SCC, parity and lactation stage on dissimilarities of microbial composition among samples using PERMANOVA. The result suggested that SCC has little impact on Bray-Curtis dissimilarity at almost all classification levels (p>0.05). Moreover, parity and lactation stage also accounted for little of the variation on overall microbial composition (Table 1). Principal coordinate analysis also showed no clustering tendency within either group and that the discrepancy between MS and LS samples remained unclear at any classification level (Supplementary Figure S2).
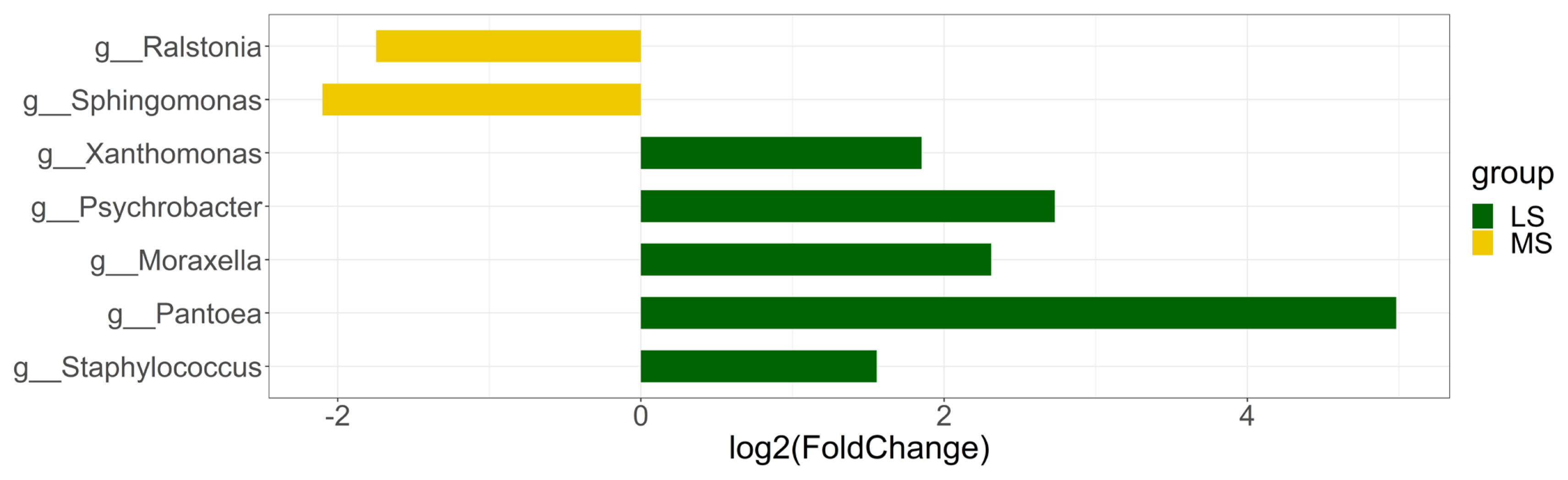
To evaluate the difference of relative abundance of specific taxon between the two groups, we utilized edgeR to normalize the counts and then identify SCC-associated taxa. Genus level was mainly focused because the difference in other lower-ranked classification levels was already captured at genus level. To further avoid the bias caused by sequencing depth, only taxa existent in at least 30% of samples in either group were kept for differential abundance analysis. Differentially abundant taxa were selected based on |log2 (fold change)| Ōēź1 and p<0.05 (Figure 3). A total of 7 genera were identified using this threshold. Two Gram-negative bacteria - Ralsotina and Sphingomonas were enriched in MS group while the abundance of Xanthomonas, Psychrobacter, Moraxella, Pantoea, Staphylococcus was significantly higher in LS group.
In order to find out whether certain host genes could potentially get involved in the interplay between bacteria and host, a combined analysis of DEG and weighted gene co-expression network was carried out to evaluate the correlation between host phenotype (SCC), gene expression pattern and the relative abundance of DAT. We first performed a DEGs analysis in MS and LS group. DEGs were identified with |log2 (fold change)| Ōēź1 and p<0.05. In general, 355 genes were differentially expressed in the two groups, of which 297 genes were up-regulated and 58 were down-regulated in MS compared with LS. Next we ranked genes according to MAD and the top 5000 genes were selected to be clustered into modules by WGCNA based on the correlation.
To further explore which module was potentially related to DAT, relative abundance of each seven differentially abundant genera identified above was used as a trait and the correlation between it and each module eigengene was evaluated. Four (M10, M11, M13, M14) out of 14 modules exhibited significant relevance to the relative abundance of Ralsotina and Sphingomonas, which were enriched in MS group (Figure 4a). A total of 297 up-regulated DEGs were mapped to genes in these four modules to find genes that were both related to SCC and MS-enriched bacteria. 0, 21, 2, 2 genes in M10, M11, M13, M14 overlapped with those in DEGs, respectively (Supplementary Figure S3). Relatively high overlap between genes in M11 and DEGs provided evidence that genes in M11 might play a role in host-microbiota interaction.
To get functional interpretation of these genes and pathways they were involved in, we conducted GO and KEGG pathways enrichment analysis. Because of the inadequate number of overlapping genes to complete enrichment analyses, an overlap between GO and KEGG pathways enriched by genes in M11 and DEGs were investigated instead. As shown in Figure 4b, approximately nine KEGG pathways were shared by both sets. Several pathways related to inflammation and immune response were identified, such as phagosome, cytokine-cytokine receptor interaction, interleukin-17 (IL-17) signaling pathway, chemokine signaling pathway, apoptosis. The overlapping GO terms that fell in biological process categorical included chemokine-mediated signaling pathway, antimicrobial humoral immune response, neutrophil chemotaxis, which were also associated with immune signals, suggesting that there was an increase in the immune system activities in MS group compared with LS group and were consistent with the physiological process during subclinical mastitis, because subclinical mastitis was usually characterized by active immune responses (Supplementary Figure S4). Equally important is that the result also demonstrated a higher level of kinase and phosphatases activities in MS which was typically involved in initiation of immune cells activation in response to antigens. Despite the fact that none of the 21 genes shared by DEGs and M11 was enriched in these pathways or terms, the result infers that the intensity of immune responses varied from subclinical mastitis cows to healthy cows, and also from cows with higher abundance of Ralstonia and Sphingomonas to cows with lower abundance of those, though the variation might not be obvious. However, one gene, F11R was found to be involved in two different enriched KEGG pathways. F11R was significantly enriched in tight junction using DEGs for pathway enrichment analysis and leukocyte transendothelial migration using M11 genes, indicating its role in initiation of inflammatory immune response and barrier function maintenance. To further determine a relevance between the change in expression level of some specific genes and the relative abundance of Ralstonia and Sphingomonas, Pearson correlation was used to find genes directly linked to these two kinds of bacteria (Figure 4d). The results of correlation test showed that transcobalamin 1 (TCN1) and uridine phosphorylase 1 (UPP1) had a strong correlation with either Ralstonia (0.78, 0.69, respectively) or Sphingomonas (0.85, 0.76, respectively).
Based on the functional annotation of metagenome, we sought to detect the biological processes that either intensified or weakened in MS group. Differentially enriched GO terms determined by edgeR are shown in Figure 5, of which four were less abundant in MS compared with LS, and 18 showed the opposite tendency. The four down-regulated GO in MS were organic hydroxy compound metabolic process, transmembrane transporter activity, kinase activity and response to organic substance. Notably, lipopolysaccharide (LPS) biosynthetic and metabolic process, response to oxygen levels and hypoxia were up-regulated. It is noteworthy that the activities of some enzymes involved in DNA replication and nucleotide synthesis also increased in MS, suggesting active bacterial reproduction was underway.
Though, previous studies have identified lots of candidate genes and potential microbial biomarkers associated with subclinical mastitis, however, to our knowledge this is the first study to combine bovine transcriptome and milk metagenome to explore the connections of host and microbiota in dairy cattle subclinical mastitis.
The present study identified a difference in the relative abundance of Ralstonia and Sphingomonas between MS and LS group. Previous studies reported association of Ralstonia with mastitis and subacute mastitis in human [9] and detected both Ralstonia and Sphingomonas as main components of donkey milk [10,11]. Ralstonia is well known to come from environmental resources and was reported to be associated with water contamination and it shows greater adaptability to adverse conditions than many other bacteria [4,12]. Same as Ralstonia, Sphingomonas are also environmental bacteria and can be found in tap water or soil. Notably, two species belonging to Sphingomonas genus (S. paucimobilis and S. maltophilia) were isolated from milk samples of clinical mastitis cows [13]. Another milk microbiota profiling study reported that Sphingomonas was more enriched in culture negative clinical milk samples, whereas, Ralstonia was more easily detected in the microbiota of healthy quarters [4]. Though, no direct association was identified between the presence of the two bacteria and clinical or subclinical mastitis, however, these were reported to be related with some human diseases, such as colitis associated cancer [14], cystic fibrosis [15] and gastric inflammation [16]. Besides, opportunistic infection can sometimes be caused by non-pathogenic bacteria.
Common KEGG pathways and GO enriched by genes in modules highly correlated with Ralstonia and Sphingomonas and genes in DEGs were associated with immune responses. Apart from that, a significantly positive correlation between the two bacteria and expression level of TCN1 and UPP1 was determined in this study (p<0.05). TCN1 encodes cobalamin (a vitamin B12-binding protein) and UPP1 is the coding gene of uridine phosphorylase 1. High expression of TCN1 and UPP1 was present in many malignant cancers. TCN1 was associated with breast phyllodes tumors [17] and was noticed to be a negative indicator in prognostic evaluation of rectal cancer [18]. High expression of UPP1 was reported in breast cancer and thyroid cancer cells [19]. Keeping in view the combined role of cobalamin and UPP1 in cell metabolism, the up-regulation of TCN1 and UPP1 in MS was reasonable, due to its involvement in a wide range of basic biological processes.
Metagenomic functional annotation results further demonstrated active host-microbe interplay in MS group. For Gram-negative bacteria, LPS is an important component of outer membrane and plays an essential part in host-pathogen interaction during infection [20]. Similarly, molecular oxygen exhaustion has been proved to be in relevance to some pathological states, such as inflammation and bacterial infection. This was also consistent with the result that hypoxia inducible factor (HIF-1) signaling pathway was significantly enriched by host genes in M11 and DEGs. The findings of the present study are in line with the previous study showing that the therapeutic effect of alpha-linolenic acid based intra-mammary nano-suspension on LPS induced mastitis in rat resulted in suppression of the HIF-1╬▒ [21]. Contrary to previous studies [5,6], our results showed that no distinct differences of global diversity and richness existed between the microbial communities in subclinical mastitis cows and in healthy ones due to great variation within group. However, we can see most samples in LS had a higher index, implying greater microbiota diversity in healthy milk samples. Similar finding in beta-diversity pattern further illustrated that there was no clear separation in overall microbial composition in different groups. Besides, sample distance ŌĆō variable association assessed by PERMANOVA demonstrated that none of the three variables (SCC, parity, and lactation) can perfectly explain the dissimilarity. This was probably because the SCC of cows we sampled was relatively concentrated and phenotype such as parity or lactation stage is unlikely to have a huge impact on composition of microbial community since it is usually in a dynamic balance [22]. Another important reason that may account for this phenomenon is the relatively inadequate depth of our metagenome sequencing to cover some rare bacteria species, as microbiota richness is sensitive to perturbation with rare bacteria [23].
In this study, metagenome sequencing was implemented instead of 16S rRNA gene sequencing, due to its advantage in gene function annotation. However, host contamination caused by somatic cells in milk posed a huge problem for this study and led to inadequate sequencing depth for rare species identification and other functional analyses i.e. virulence factors abundance investigation. However, to maintain statistic power, comparison between groups was all based on relative abundance, a typical library size correction method in metagenomics analysis. And we set a high threshold for the minimum sample size of each group that specific bacteria or GO term needed to be present in order to be kept for subsequent comparative analysis.
Transcriptome analysis was performed based on RNA derived from peripheral blood instead of milk mainly because of an ease of RNA extraction and representativeness of peripheral blood transcriptomic signature [24]. We noticed that using 100,000 SCC/mL as a cut-off value for differentiation between healthy and subclinical mastitis is controversial in different countries [25]. Though, some studies reported that a history of bovine mastitis may also lead to changes in microbial community [26,27], however, we did not take the information into account at the beginning of this study. It is suggested to use strict criteria for selection of samples with different cut-off values and a higher sequencing depth in future study.
In conclusion, we conducted an integrated analysis of milk microbiota and peripheral blood transcriptome profiling in healthy and subclincal mastitis cows in the current study. Although the exact mechanism behind the host-microbe interaction in the development of bovine subclinical mastitis has not been fully elaborated by us, our results highlight that identifying some specific host genes expression pattern changes may aid in detecting the infection in the bovine udder.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
The authors wish to thank all the members of Molecular and Quantitative Genetics Laboratory (China Agricultural University) that may contribute to this study and Novogene Technology (Beijing, China) for the sequencing services. The financial support provided by National Natural Science Foundation of China-Pakistan Science Foundation (NSFC-PSF) Joint Project (31961143009) National Key R&D Program of China (2021YFD1200903 and Beijing Natural Science Foundation (6182021) is acknowledged.
SUPPLEMENTARY MATERIAL
Supplementary file is available from: https://doi.org/10.5713/ab.21.0495
Supplementary Figure S1. Distribution of parity, lactation days and SCC in all 31 cows.
Supplementary Figure S2. Results of Principal coordinates analysis showing the dissimilarity between samples at different classification levels.
Supplementary Figure S3. Differentially expressed genes (MS vs LS).
Supplementary Figure S4. Gene Ontology (GO) enrichment results for DEGs.
ab-21-0495-Supplementary-Figs.pdf
Figure┬Ā1
Relative abundance of top six highly representative bacteria at phylum level within each sample. MS, medium somatic cell count; LS, low somatic cell count.

Figure┬Ā2
Comparison of microbial diversity between samples in MS and LS group at different sampling depths. Chao1, Shannon, Simpson are three different diversity indices. ns, means that the difference is not significant (p>0.05). MS, medium somatic cell count; LS, low somatic cell count.
Figure┬Ā3
Genus with significant differences between MS and LS (LS vs MS). Genus enriched in MS and LS are shown in yellow bars and green bars, respectively. MS, medium somatic cell count; LS, low somatic cell count.
Figure┬Ā4
Overlap of genes correlated with differentially abundant taxa and genes differentially expressed in MS and LS. (a) Heatmap of module-trait relationship. Each row corresponds to eigengene of a module and column to the relative abundance of a differentially abundant taxa. (b) KEGG pathway enrichment results for DEGs. Pathways labeled in red represent pathways enriched by both DEGs and genes in M11. Rich factor was calculated based on the number of DEGs enriched in this pathway and the number of background genes. (c) Correlation between genes shared by both DEGs and M11 and the relative abundance of Ralstonia and Sphingomonas. Significance was established at p<0.05). ** Stands for p<0.01. (d) Map of the KEGG signaling pathways of tight junction and leukocyte transendothelial migration. Protein transcribed from F11R was marked with red asterisks. MS, medium somatic cell count; LS, low somatic cell count; KEGG, Kyoto encyclopedia of genes and genomes; DEG, differentially expressed genes.

Figure┬Ā5
Differentially enriched GO terms (MS vs LS). Significance was established using p<0.05 and |log2(fold change)| Ōēź1. Yellow bars indicate enriched GO terms in MS and green bars indicate enriched GO terms in LS. MS, medium somatic cell count; LS, low somatic cell count; GO, gene ontology.

Table┬Ā1
Assessment of variation explained by various experimental factors at different classification level using PERMANOVA
REFERENCES
1. Oikonomou G, Bicalho ML, Meira E, et al. Microbiota of cowŌĆÖs milk; distinguishing healthy, sub-clinically and clinically diseased quarters. PLoS One 2014; 9:e85904
https://doi.org/10.1371/journal.pone.0085904



2. Derakhshani H, Fehr KB, Sepehri S, et al. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J Dairy Sci 2018; 101:10605ŌĆō25.
https://doi.org/10.3168/jds.2018-14860


3. Addis MF, Tanca A, Uzzau S, Oikonomou G, Bicalho RC, Moroni P. The bovine milk microbiota: insights and perspectives from -omics studies. Mol Biosyst 2016; 12:2359ŌĆō72.
https://doi.org/10.1039/c6mb00217j


4. Kuehn JS, Gorden PJ, Munro D, et al. Bacterial community profiling of milk samples as a means to understand culture-negative bovine clinical mastitis. Plos One 2013; 8:e61959
https://doi.org/10.1371/journal.pone.0061959



5. Pang MD, Xie X, Bao HD, et al. Insights into the bovine milk microbiota in dairy farms with different incidence rates of subclinical mastitis. Front Microbiol 2018; 9:2379
https://doi.org/10.3389/fmicb.2018.02379



6. Wang Y, Nan XM, Zhao YG, et al. Coupling 16S rDNA sequencing and untargeted mass spectrometry for milk microbial composition and metabolites from dairy cows with clinical and subclinical mastitis. J Agric Food Chem 2020; 68:8496ŌĆō508.
https://doi.org/10.1021/acs.jafc.0c03738


7. Song MY, He YH, Zhou HK, et al. Combined analysis of DNA methylome and transcriptome reveal novel candidate genes with susceptibility to bovine Staphylococcus aureus subclinical mastitis. Sci Rep-Uk 2016; 6:29390
https://doi.org/10.1038/srep29390



8. Dahlberg J, Williams JE, McGuire MA, et al. Microbiota of bovine milk, teat skin, and teat canal: Similarity and variation due to sampling technique and milk fraction. J Dairy Sci 2020; 103:7322ŌĆō30.
https://doi.org/10.3168/jds.2019-17783


9. Patel SH, Vaidya YH, Patel RJ, et al. Culture independent assessment of human milk microbial community in lactational mastitis. Sci Rep-Uk 2017; 7:7804
https://doi.org/10.1038/s41598-017-08451-7



10. Soto Del Rio MLD, Dalmasso A, Civera T, Bottero MT. Characterization of bacterial communities of donkey milk by high-throughput sequencing. Int J Food Microbiol 2017; 251:67ŌĆō72.
https://doi.org/10.1016/j.ijfoodmicro.2017.03.023


11. Papademas P, Kamilari E, Aspri M, et al. Investigation of donkey milk bacterial diversity by 16S rDNA high-throughput sequencing on a Cyprus donkey farm. J Dairy Sci 2021; 104:167ŌĆō78.
https://doi.org/10.3168/jds.2020-19242


12. Ryan MP, Pembroke JT, Adley CC. Genotypic and phenotypic diversity of Ralstonia pickettii and Ralstonia insidiosa isolates from clinical and environmental sources including High-purity Water. Diversity in Ralstonia pickettii. BMC Microbiol 2011; 11:194
https://doi.org/10.1186/1471-2180-11-194



13. Nam HM, Lim SK, Kang HM, et al. Prevalence and antimicrobial susceptibility of gram-negative bacteria isolated from bovine mastitis between 2003 and 2008 in Korea. J Dairy Sci 2009; 92:2020ŌĆō6.
https://doi.org/10.3168/jds.2008-1739


14. Richard ML, Liguori G, Lamas B, et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 2018; 9:131ŌĆō42.
https://doi.org/10.1080/19490976.2017.1379637



15. Green HD, Bright-Thomas R, Kenna DT, Turton JF, Woodford N, Jones AM. Ralstonia infection in cystic fibrosis. Epidemiol Infect 2017; 145:2864ŌĆō72.
https://doi.org/10.1017/S0950268817001728



16. Sung JJY, Coker OO, Chu E, et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020; 69:1572ŌĆō81.
https://doi.org/10.1136/gutjnl-2019-319826



17. Chong LYZ, Cheok PY, Tan WJ, et al. Keratin 15, transcobalamin I and homeobox gene Hox-B13 expression in breast phyllodes tumors: novel markers in biological classification. Breast Cancer Res Treat 2012; 132:143ŌĆō51.
https://doi.org/10.1007/s10549-011-1555-6


18. Lee YY, Wei YC, Tian YF, et al. Overexpression of transcobalamin 1 is an independent negative prognosticator in rectal cancers receiving concurrent chemoradiotherapy. J Cancer 2017; 8:1330ŌĆō7.
https://doi.org/10.7150/jca.18274



19. Guan YY, Bhandari A, Zhang XH, Wang OC. Uridine phosphorylase 1 associates to biological and clinical significance in thyroid carcinoma cell lines. J Cell Mol Med 2019; 23:7438ŌĆō48.
https://doi.org/10.1111/jcmm.14612



20. Maldonado RF, Sa-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev 2016; 40:480ŌĆō93.
https://doi.org/10.1093/femsre/fuw007



21. Yadav RK, Tripathi CB, Saraf SA, et al. Alpha-linolenic acid based nano-suspension protect against lipopolysaccharides induced mastitis by inhibiting NFkappaBp65, HIF-1alpha, and mitochondria-mediated apoptotic pathway in albino Wistar rats. Toxicol Appl Pharmacol 2019; 377:114628
https://doi.org/10.1016/j.taap.2019.114628


22. Fernandez MF, Reina-Perez I, Astorga JM, et al. Breast cancer and its relationship with the microbiota. Int J Environ Res Public Health 2018; 15:1747
https://doi.org/10.3390/ijerph15081747



23. Rault L, Leveque PA, Barbey S, et al. Bovine teat cistern microbiota composition and richness are associated with the immune and microbial responses during transition to once-daily milking. Front Microbiol 2020; 11:602404
https://doi.org/10.3389/fmicb.2020.602404



24. Basu M, Wang K, Ruppin E, Hannenhalli S. Predicting tissue-specific gene expression from whole blood transcriptome. Sci Adv 2021; 7:eabd6991
https://doi.org/10.1126/sciadv.abd6991



25. Schwarz D, Diesterbeck US, Konig S, et al. Microscopic differential cell counts in milk for the evaluation of inflammatory reactions in clinically healthy and subclinically infected bovine mammary glands. J Dairy Res 2011; 78:448ŌĆō55.
https://doi.org/10.1017/S0022029911000574


26. Falentin H, Rault L, Nicolas A, et al. Bovine teat microbiome analysis revealed reduced alpha diversity and significant changes in taxonomic profiles in quarters with a history of mastitis. Front Microbiol 2016; 7:480
https://doi.org/10.3389/fmicb.2016.00480



27. Metzger SA, Hernandez LL, Skarlupka JH, et al. A cohort study of the milk microbiota of healthy and inflamed bovine mammary glands from dryoff through 150 days in milk. Front Vet Sci 2018; 5:247
https://doi.org/10.3389/fvets.2018.00247



- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print





