 |
 |
| Anim Biosci > Volume 33(3); 2020 > Article |
|
Abstract
Objective
Ensiling is a simple and effective method for long-term preservation; however, less information exists about the ensilability characteristics of garlic stalk (GS). Therefore, the objectives were to examine the ensiling feasibility of GS.
Methods
The GS was ensiled alone or inoculated with Lactobacillus plantarum KU5 in the presence or absence of 5% molasses and ensiled for 7, 14, and 28 d. As an alternative storage method, GS was co-ensiled with wet citrus pulp (CP) at different proportions (GS:CP: 70:30, 60:40, 50:50, and 40:60). Analysis was made on physicochemical, fermentative, and nutritional parameters.
Results
The GS was found to be a biomass which is difficult to ensile. A combination of microbial inoculant and molasses was successful in the improvement of the silage fermentation quality of GS. Co-ensiling of GS with wet CP at the mixing ratio of 50:50 provided the most desirable silage fermentation parameters, including the substantial lactic acid formation, low final pH, minor effluent loss, and the more favorable organoleptic properties.
The worldwide production of garlic amounted to 27 million tons in 2016, with China, India, and Korea being the major garlic producing countries [1]. Nearly 70% of the total garlic product is discarded as stalk, which could be used as a potential roughage source in a ruminant diet [2,3]. However, during the harvesting season large quantities of garlic stalk (GS) accumulate, which exceeds the amount needed for immediate use. Thus, the surplus is simply decomposed into the soil or incinerated, the latter resulting in environmental concerns [2,4]. This demands the development of an efficient conservation technology for its long-term use.
The ensiling process is an effective and simple conservation technology that is known to acidify biomass, thus it inhibits the growth of spoilage-causing microorganisms, thereby providing safe and long-term conservation for moist forages [5]. Inoculation with lactic acid bacteria (LAB) or supplementation with readily fermentable carbohydrates (readily metabolizable by LAB), such as molasses, proved to be successful in enhancing the efficiency of the ensiling process. This supplies an additional source of simple sugars for the growth and multiplication of LAB, thereby accelerating the silage acidification and avoiding microbial deterioration, especially when the ensiling biomass has a low concentration of soluble carbohydrates [5,6]. More recently, the simultaneous application of the LAB inoculant and molasses successfully improved the silage fermentation quality of various crops [7,8].
Citrus is one of the major fruits in Korea, which is mainly cultivated on Jeju Island and the southern coast line of the main land, with an estimated production of 0.68 million tons in 2011 [9]. An estimated 30% of citrus fruits is directed into the processing industries, resulting in the generation of about 50% to 60% organic waste [10]. Citrus pulp (CP), the main by-product of citrus processing facilities, is rich in the fermentable carbohydrates but high in moisture content (approximately 80% to 90%), which is associated with difficulties in its transportation, handling, and perishability [11,12]. Moreover, the high moisture content of CP results in effluent loss during ensiling, which is associated with the loss of silage nutrients and environmental worries arising from the pollution of surface and ground water [13]. Additionally, the high residual sugars give CP a wet and sticky nature that raises issues associated with its conservation in silos [12]. Earlier studies mainly investigated the dried CP as animal feed; however, drying is an expensive process and therefore an improper method for large-scale recycling of CP [12]. However, the appropriate mixing of CP with dry ingredients, to maintain the proper moisture content for silage making, would contribute to the efficient recycling of high-moisture CP [11]. Therefore, we hypothesized that combining GS (as a drier by-product) and CP could enhance the silage fermentation quality of the two by-products.
To our knowledge, limited reports are available concern ing the silage fermentation characteristics of GS. Therefore, the first objective was to evaluate how Lactobacillus plantarum (L. plantarum) inoculation without or with molasses would affect the silage fermentation patterns of GS. The second objective was to develop an alternative storage method through co-ensiling of GS with CP at different proportions, and determine the appropriate mixing ratio for efficient silage fermentation.
Different batches of sun-dried GS were provided from a garlic processing plant located in Uysung County, Kyung-Buk province and an agricultural marketing center located in Chungju city, Chung-Buk province in Korea. Before silage making, GS was cut into pieces of 20 to 30 mm and hand-mixed thoroughly, then divided into the respective treatments. L. plantarum KU5 (Accession No. HQ542227) inoculant was re-cultivated for 3 consecutive days on MRS (de Mann Rogosa Sharpe) broth (Difco Laboratories Inc., Detroit, MI, USA) at 36°C for 24 h, before being applied onto the biomass.
The GS was ensiled in nylon-polyethylene pouches (5 replicates per each treatment) without inoculant or with L. plantarum KU5 (LAB) in the absence or presence of % molasses (LAB+ M). Ensiling duration was 7, 14, and 28 d at room temperature (20°C to 24°C). The detailed description of the silage making procedure is reported in our previous article [8]. The suspension containing the LAB inoculant was applied onto the mixture using a manual sprayer at a rate to provide 1×106 cfu/g of fresh mass. In a subsequent experiment, GS was mixed with CP at decremental proportions of 70:30, 60:40, 50:50, and 40:60 (wet weight), mixed manually, and then assigned to respective treatments. The packing density of approximately 200 kg dry matter (DM)/m3 was applied for all silages, before the sealing of the polyethylene silos. From the designated silo openings, the representative samples were collected randomly for the subsequent analyses.
Contents of DM, crude protein (N×6.25), ether extract, crude ash, neutral-detergent fiber (NDF), and acid detergent fiber, both corrected for residual ash, were determined according to the standard methods of the Association of Official Analytical Chemists [14]. True protein was determined after precipitation in 5% trichloroacetic acid solution [15]. Non-protein nitrogen was calculated as “100 − true protein”. Non-fibrous carbohydrate (NFC) was computed as 100 − (crude protein + NDF + ether extract + crude ash). For silage juice extraction, a 20 g sample of fresh or silage biomass was mixed with deionized autoclaved water (200 mL) and blended (DIAX 900, Heidolph, Schwabach, Germany) for 1 min. The suspension was filtered through two layers of medical gauze, and was used for the measurement of pH (HI 9321, Hanna Instruments, Woonsocket, RI, USA), water-soluble carbohydrates (WSC, glucose equivalent) [16], lactic acid [17], and NH3-N [18] using a UV-visible spectrophotometer (S-1100, Scinco, Daejeon, Korea). The viable colonies of total bacteria and LAB were counted using the spread-plating method on plate count agar and MRS agar, respectively. Plates were incubated (JSGI-050T, Hanyang Scientific Equipment Co., Ltd., Seoul, Korea) at 36°C for 48 h.
Data analyses were performed using the general linear model procedure (Statistix7, 2000). Treatment effects were evaluated with the analysis of variance in a completely randomized design. Each mini-silo was considered as the experimental unit. Microbial counts were transformed to log10-scale before statistical analysis. The difference among treatments was identified using Tukey’s multiple range test. Values less than 0.05 probability level were considered significant. Tendency was discussed at 0.05<p<0.1.
The silage fermentation metabolites and chemical composition of GS ensiled for 7, 14, or 28 d, are presented in Table 1. Initial pH before ensiling was 8.06, which declined (p<0.01) as the length of ensiling prolonged (a 1.26-unit decline by d 28 of ensiling). Lactic acid concentration was greatest after 28 d of fermentation. This is associated with the development of LAB which tended to increase (p = 0.07) as fermentation duration continued. With ensiling, WSC content decreased while NH3-N formation increased considerably (a 4.4-times increase after 28 d of ensiling), which indicates the microbial conversion of WSC into lactic acid and protein breakdown during ensiling, respectively [5,19]. The chemical composition of GS silage differed negligibly as ensiling duration progressed. Crude protein content declined slightly with ensiling (p< 0.002), which can possibly be explained by the protein breakdown (proteolysis) during ensiling, which releases NH3-N [5].
An approximate number of 8 log 10 cfu of LAB per gram of fresh biomass is the required LAB number to ensure the fast decline of silage pH [20]. In the present experiment, although the population of epiphytic LAB was adequate (7.93 log10 cfu/g) to promote fast acidification and efficient ensiling, the silage fermentation was not successful, as evidenced by high NH3-N content and high silage pH after 28 d of fermentation. This suggests that GS is a difficult-to-ensile biomass. This can be justified by three explanations: i) The GS contains an inadequate content of WSC, which probably did not provide sufficient sugars for lactic acid production, and thus silage mass acidification [5,6]. ii) The GS contains a high ash concentration (approximately 15%), which would increase the buffering capacity, thus resisting silage mass acidification [5]. iii) GS has a hollow and tubular structure [4] that increases the porosity of GS mass, accelerates the aerobic microbial activity during the early phase of ensiling, and thus converts readily available carbohydrates into carbon dioxide and water [5]. These factors collectively result in an inefficient anaerobic fermentation and slow acidification [20]. Based on these assumptions, the follow-up experiment attempted to improve the fermentation quality of GS with LAB inoculation in the presence of molasses as a source of WSC.
The silage fermentation dynamics of GS inoculated with LAB in the absence or presence of molasses in relation to the length of ensiling (7, 14, and 28 d), are presented in Table 2. Generally, the fermentation quality parameters of GS silage treated with a combination of LAB+M were preferable to those of untreated or LAB-treated silages. After 7 d, silage pH declined from the initial value of 7.7 in the control silage to 5.8 in the LAB+M silage, indicating that the combination of LAB and molasses promoted the favorable silage fermentation pattern.
Inoculation with LAB prior to ensiling has been successful in the promotion of desirable fermentation patterns. However, when the epiphytic population of LAB exceeds the inoculant application rate, the domination of inoculant bacteria in the silage is difficult [21]. In the present experiment, the natural population of LAB in GS was close to 8 log cfu/g of fresh GS (prior to ensiling), which considerably exceeded the LAB application rate (106 cfu/g of fresh GS). Surprisingly, LAB+M treatment promoted the more favorable fermentative patterns (with respect to a lower pH and less NH3-N production) than untreated or LAB-treated silage. After 28 d of ensiling, LAB+M-treated silage showed a 35% increase in lactic acid content and a 0.9-unit decline in pH, with respect to the LAB-inoculated silage. In support, Huisden et al [19] reported that the addition of molasses to corn silage supplied an extra source of WSC for LAB metabolism, which possibly allowed their domination in the microbial community of the silage, thereby stimulating favorable silage fermentation patterns.
Earlier findings confirmed that the combined use of the LAB inoculant and molasses improved the silage quality indices of various crops [7,8,22]. For example, our recent investigation [8] found that the combination of LAB and 5% molasses efficiently improved the silage quality parameters of spent mushroom substrate in both laboratory-scale and ton-bag silos. Presently, when GS was ensiled without molasses or a microbial inoculant, silage pH slightly declined (6.80 after 28 d of ensiling), which was accompanied by the high NH3-N accumulation (631 ppm). However, when GS was ensiled with LAB+M, the silage pH dropped to 5.3 (after 28 d of ensiling) and as anticipated, less NH3-N was formed (Table 2). This observation was expected, because when silage pH declines more quickly, excessive proteolysis is suppressed during ensiling, which contributes to less NH3-N formation [5].
The chemical composition of GS ensiled with LAB in the absence or presence of molasses in relation to the length of ensiling, is presented in Table 3. No difference in the chemical composition was seen after 7 d of ensiling. However, as ensiling duration prolonged, the NDF concentration decreased in LAB+M-treated silage, which is possibly associated with the hydrolysis of cell wall fractions that are more digestible during ensiling fermentation [19].
This experiment evaluated the co-ensiling of GS with CP as an alternative storage technology, which has been successful in improving the silage fermentation quality of several feedstuffs [23]. For example, the recent co-ensiling of wheat straw with sugar beet waste has proved to be a successful storage method for their long-term preservation [24]. The typical silage quality parameters and chemical composition of GS and CP, ensiled alone or together (GS 70%+CP 30%) after 7, 14, or 28 d of ensiling, are reported in Tables 4 and 5, respectively. Generally, GS ensiled with CP exhibited more desirable fermentation characteristics in comparison to GS silage alone. For example, after 14 d of ensiling, the GS 70%+CP 30% silage exhibited a 56% increase in lactic acid content which represented a 0.4-unit decline in silage pH, with respect to GS ensiled alone. The nutrient composition of GS 70%+CP 30% silage was comparable to that of GS ensiled alone. However, after 14 and 28 d of ensiling, a greater NFC concentration was recorded for GS 70%+CP 30% silage, which is associated with an NDF content that diminished as ensiling time continued. For example, after 28 d of ensiling, NDF concentration decreased by 7.5% with respect to GS silage, which was possibly caused by the dissolution of the more digestible NDF fractions during ensiling [19]. These findings showed promise in the possibility of the successful preservation of GS when co-ensiled with CP, leading to the follow-up experiments to determine the proper mixing level of GS and CP for efficient silage fermentation.
The pH of GS co-ensiled with incremental proportions of CP is illustrated in Figure 1. After 14 d of ensiling, the pH of GS 50%+CP 50% silage declined markedly, which is suggestive of successful silage fermentation that is known to slow down or inhibit the growth of fungi, molds, and yeasts [25]. Consistent with our findings, Migwi et al [23] reported that when 5% molasses was added to the mixture of wheat straw+ broiler litter, no effect on silage pH was detected. However, when the proportion of CP increased from 0 to 30% in the silage, the pH declined markedly [23]. Similarly, when CP was ensiled alone, lactic acid concentration was negligible (1.02% of DM, after 14 d of ensiling; Figure 2). However, when CP was co-ensiled with GS at a 50:50 proportion, lactic acid production increased markedly (6.0% of DM, after 14 d of ensiling).
The silage fermentation, sensory, and physical parame ters of GS co-ensiled with incremental proportions of CP are shown in Table 6. After 2 weeks of fermentation, despite the substantial increase in lactic acid production with increasing CP proportion (Figure 2), total viable colonies of LAB remained unaffected with increasing proportions of CP in the silage. This might be explained by the counting technique used, as this requires actively growing cells. It has been proposed that many LAB exist on the biomass surfaces that are viable but not culturable by using the traditional plating methods [26]. Assuming this proposition, it appears that the increased availability of metabolic water provided by the increased proportion of CP into GS biomass supported the higher metabolic activity of the microbial ecology of silage, particularly LAB [27]. In agreement, Muck [28] ensiled alfalfa at different DM levels (17% to 73%) and found that the greatest levels of lactic acid were produced in silages of 40% to 55% DM. Likewise, Mthiyane et al [29] found that when water was added to sugarcane tops silage, lactic acid production was restricted, which was explained by the multiplication and domination of heterofermentative LAB in high-moisture silage, as this generates the mixed metabolites of acetic acid, ethanol, and lactic acid. Essentially, silage making at the optimal moisture level should provide enough moisture for LAB growth and metabolism to decrease pH and prevent the growth of putrefactive microorganisms, while also providing enough dryness to minimize effluent production [5,13]. Presently, it appears that the 50:50 mixing proportion provided the appropriate moisture level that favored the metabolic activity of LAB in the mixed silage of GS and CP, and thus promoted substantial lactic acid production. However, the reasons why lactic acid production increased with moisture alterations of the silage are still not clear and necessitate more studies to identify the contributory factors to this observation.
As the GS:CP mixing proportion decreased, NH 3-N concentration lessened, which is indicative of diminished proteolysis and deamination during ensiling [5]. After 14 d of ensiling, NH3-N content accounted for 25.4% of the total N in GS 50% +CP 50% silage, which is approximately 70% less than the silages with 30% or 40% CP. The fast acidification of silage mass suppresses the growth of putrefying microorganisms, this probably occurred as the mixing ratio of GS:CP decreased, resulting in a lower formation of NH3-N, which is associated with the improved utilization of silage-N and thus microbial protein synthesis in the rumen [20]. Decreasing the porosity of forage silage mass using supplementary feed sources, such as corn meal, with fine particle sizes may accelerate the silage pH decline, diminish inefficient plant respiration (less heat generation), and thus promote effective anaerobic fermentation [30]. This series of events is known to minimize proteolysis, which is accelerated at high silage pH and temperature [20]. It appears that the co-mixing of CP and GS decreased the porosity of the GS mass, which thereby promoted efficient silage fermentation that minimized NH3-N formation.
No putrid odor or moldy spots were detected in the ex perimental silages (Table 6), which suggests that the silages underwent a successful fermentation. However, fermentation odor increased and garlic odor decreased as the incremental levels of CP were incorporated into GS silage. The absorption degree was highest when 40% or 50% CP was incorporated into GS, which indicates that the effluent lost from CP was efficiently assimilated into GS biomass. This finding is of great importance as CP has a high moisture content and its ensiling alone would cause problems associated with effluent and silage nutrient losses [12], which can be minimized when co-ensiled with GS at the proper mixing ratio. Overall, this experiment found that when GS and CP were combined at a 50:50 proportion, the best ensiling and physical properties were achieved, as demonstrated by the domination of LAB, low final pH, substantial lactic acid formation, and negligible effluent loss, thus opening an avenue for the simultaneous and long-term conservation of these waste residues.
The chemical composition of GS co-ensiled with incremen tal proportions of CP is shown in Table 7. As the proportion of CP increased in the silage mass the DM, NDF, and ether extract contents declined, and NFC content increased as expected. After 14 d of ensiling, GS 50%+CP 50% silage resulted in a 6.9% reduction in NDF content, with respect to GS ensiled with 30% CP, resulting in a higher NFC concentration, which is suggestive of the improved feed-nutritional quality of the mixed silage of GS and CP for ruminant feed.
A huge amount of GS is generated during the harvesting season, an amount considerably exceeding the immediate use. This surplus is mainly composted into soil or incinerated, which is associated with serious environmental concerns. The simultaneous application of L. plantarum inoculant and molasses favorably promoted the silage fermentation quality of GS. As an alternate storage method, the effective silage fermentation was also achieved when GS was co-ensiled with CP at the mixing ratio of 50:50. Co-ensiling of GS with CP appears to be a simple and feasible method of preservation, which would provide the more efficient utilization of these waste resources over longer periods, especially in places where they are generated in large quantities.
Figure 1
Changes in silage pH of garlic stalk co-ensiled with citrus pulp at varying proportions. ■, 0 d of ensiling; □, after 14 d of ensiling. a–c Means with different letters within the same line differ (p<0.05). Error bars at each point represent standard error.

Figure 2
Lactic acid concentration of garlic stalk co-ensiled with citrus pulp at varying proportions. ■, 0 d of ensiling; □, after 14 d of ensiling. a–c Means with different letters within the same line differ (p<0.05). Error bars at each point represent standard error.
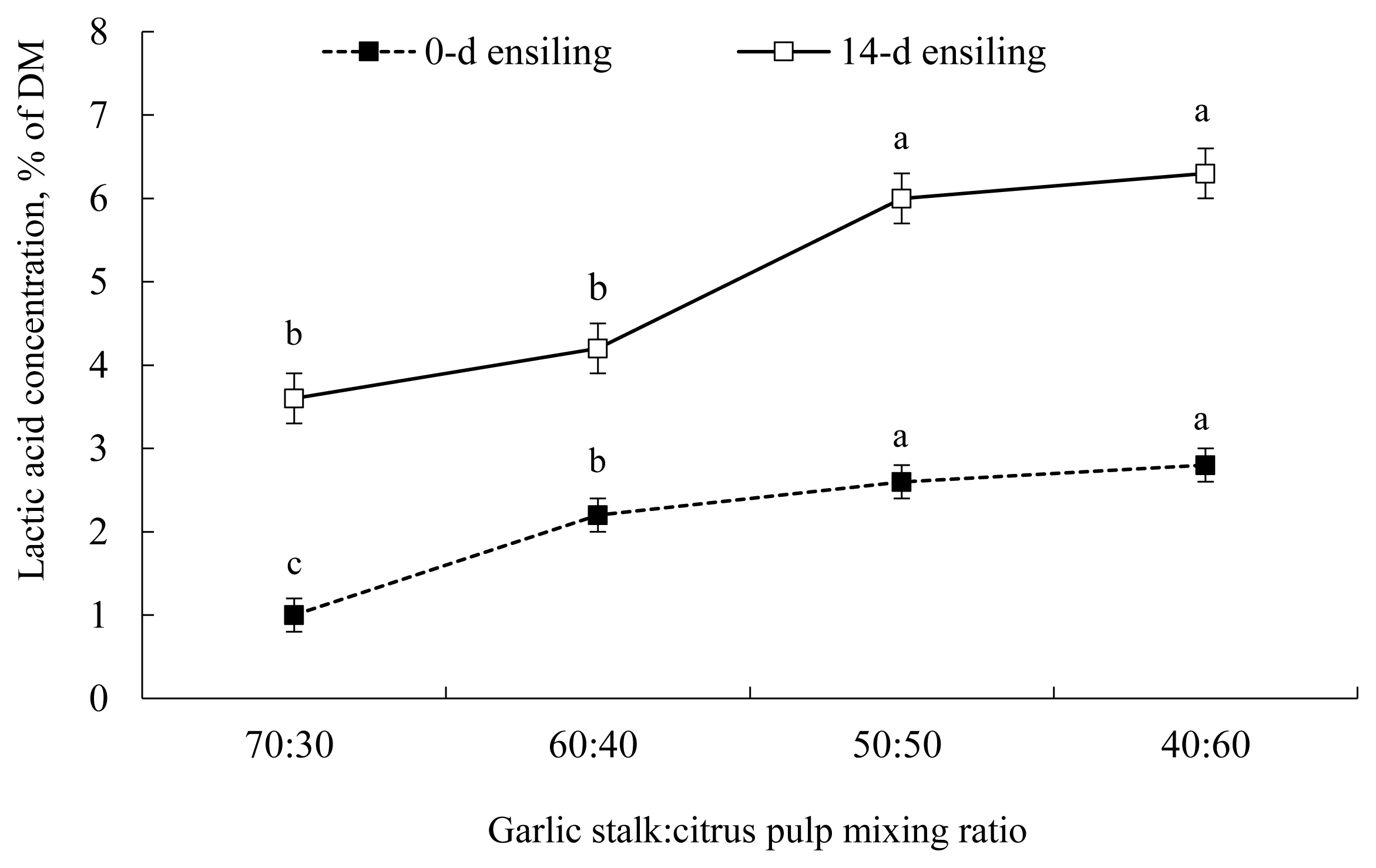
Table 1
Silage quality parameters and chemical composition of garlic stalk in relation to the ensiling duration
| Items | Ensiling duration (d) | SE | p-value | |||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 7 | 14 | 28 | |||
| Silage quality parameters | ||||||
| pH | 8.06c | 7.68b | 7.69b | 6.80a | 0.28 | <0.001 |
| WSC (% of DM) | 2.10a | 1.51b | 1.92a | 1.44b | 0.20 | 0.021 |
| Lactic acid (% of DM) | 0.42a | 0.40a | 0.42a | 0.49b | 0.03 | 0.013 |
| NH3-N (ppm) | 143a | 311b | 424b | 631c | 24.8 | <0.001 |
| Lactic acid bacteria, log10 cfu/g1) | 7.93 | 8.36 | 8.15 | 8.23 | 0.13 | 0.069 |
| Chemical composition | ||||||
| Dry matter (%) | 63.2 | 64.4 | 63.5 | 62.8 | 0.81 | 0.325 |
| Crude protein (% of DM) | 7.02a | 7.01a | 6.40b | 6.32b | 0.20 | 0.002 |
| True protein (% of crude protein) | 57.8 | 52.8 | 56.2 | 52.8 | 1.31 | 0.123 |
| NPN (% of crude protein) | 42.2 | 47.2 | 43.8 | 47.2 | 1.33 | 0.123 |
| NDF (% of DM) | 49.7 | 50.4 | 48.8 | 49.4 | 1.41 | 0.532 |
| ADF (% of DM) | 48.4 | 49.9 | 50.2 | 48.9 | 1.54 | 0.806 |
| Ether extract (% of DM) | 1.73 | 1.58 | 1.57 | 1.70 | 0.21 | 0.974 |
| Crude ash (% of DM) | 15.9 | 15.5 | 16.9 | 16.7 | 0.34 | 0.322 |
Table 2
Effects of microbial inoculant without or with molasses on the silage quality parameters of garlic stalk after 7, 14, and 28 d of ensiling
| Items | Treatment1) | SE | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| Control | LAB | LAB+M | |||
| Ensiled for 7 d | |||||
| pH | 7.7a | 7.5a | 5.8b | 0.31 | 0.001 |
| WSC (% of DM) | 1.5c | 2.0b | 2.4a | 0.23 | 0.002 |
| Lactic acid (% of DM) | 0.40 | 0.42 | 0.44 | 0.02 | 0.298 |
| NH3-N (ppm) | 311 | 349 | 284 | 27.9 | 0.116 |
| Lactic acid bacteria, log10 cfu/g2) | 8.4 | 8.8 | 8.8 | 0.20 | 0.107 |
| Ensiled for 14 d | |||||
| pH | 7.7a | 7.7a | 5.4b | 0.30 | <0.001 |
| WSC (% of DM) | 1.9ab | 1.7b | 2.3a | 0.21 | 0.009 |
| Lactic acid (% of DM) | 0.42b | 0.42b | 0.64a | 0.07 | 0.027 |
| NH3-N (ppm) | 424ab | 502a | 362b | 30.9 | 0.006 |
| Lactic acid bacteria, log10 cfu/g | 8.2b | 8.4b | 8.9a | 0.11 | 0.001 |
| Ensiled for 28 d | |||||
| pH | 6.8a | 6.3ab | 5.3b | 0.31 | 0.042 |
| WSC (% of DM) | 1.4 | 1.4 | 1.7 | 0.10 | 0.050 |
| Lactic acid (% of DM) | 0.49 | 0.54 | 0.66 | 0.06 | 0.070 |
| NH3-N (ppm) | 631 | 553 | 475 | 56.9 | 0.071 |
| Lactic acid bacteria, log10 cfu/g | 8.2 | 8.4 | 8.5 | 0.24 | 0.230 |
Table 3
Effects of microbial inoculant without or with molasses on the chemical composition of garlic stalk after 7, 14, and 28 d of ensiling
| Items | Treatment1) | SE | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| Control | LAB | LAB+M | |||
| Ensiled for 7 d | |||||
| Dry matter (%) | 64.4 | 63.3 | 63.9 | 0.73 | 0.356 |
| Crude protein | 7.0 | 7.1 | 7.0 | 0.31 | 0.868 |
| True protein (% of crude protein) | 52.8 | 53.4 | 52.2 | 1.60 | 0.751 |
| NPN (% of crude protein) | 47.2 | 46.6 | 47.8 | 1.61 | 0.751 |
| Crude ash | 15.5 | 15.4 | 15.2 | 0.32 | 0.666 |
| NDF | 50.4 | 51.7 | 51.4 | 1.30 | 0.603 |
| ADF | 49.9 | 49.7 | 48.4 | 1.21 | 0.448 |
| Ensiled for 14 d | |||||
| Dry matter (%) | 63.5 | 63.1 | 61.5 | 1.14 | 0.191 |
| Crude protein | 6.4b | 6.6b | 7.3a | 0.20 | 0.012 |
| True protein (% of crude protein) | 56.2a | 52.2ab | 48.4b | 1.71 | 0.004 |
| NPN (% of crude protein) | 43.8b | 47.8ab | 51.6a | 1.71 | 0.004 |
| Crude ash | 16.9 | 16.1 | 16.7 | 0.81 | 0.560 |
| NDF | 48.8ab | 49.9a | 46.1b | 1.33 | 0.036 |
| ADF | 50.2 | 48.9 | 47.7 | 1.31 | 0.194 |
| Ensiled for 28 d | |||||
| Dry matter (%) | 62.8 | 61.2 | 63.1 | 0.83 | 0.067 |
| Crude protein | 6.3 | 6.8 | 6.7 | 0.21 | 0.150 |
| True protein (% of crude protein) | 52.8 | 51.4 | 52.2 | 1.81 | 0.748 |
| NPN (% of crude protein) | 47.2 | 48.6 | 47.8 | 1.84 | 0.748 |
| Crude ash | 16.7a | 16.1a | 15.2b | 0.33 | 0.003 |
| NDF | 49.4a | 48.4a | 46.6b | 0.40 | <0.001 |
| ADF | 48.0 | 46.1 | 46.0 | 0.81 | 0.052 |
Table 4
Effects of the co-ensiling of garlic stalk with citrus pulp on the silage quality parameters after 7, 14, and 28 d of ensiling
| Items | Treatment1) | SE | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| GS 100% | CP 100% | GS 70% +CP 30% | |||
| d 0 (before ensiling) | |||||
| pH | 7.5a | 3.2c | 5.7b | 0.11 | <0.001 |
| Lactic acid (% of DM) | 0.16b | 2.45a | 0.19b | 0.09 | <0.001 |
| Ensiled for 7 d | |||||
| pH | 6.6a | 3.0c | 5.6b | 0.10 | <0.001 |
| Lactic acid (% of DM) | 0.17b | 1.84a | 0.20b | 0.10 | <0.001 |
| Ensiled for 14 d | |||||
| pH | 6.3a | 3.1c | 5.9b | 0.11 | <0.001 |
| Lactic acid (% of DM) | 0.16b | 1.02a | 0.25b | 0.10 | <0.001 |
| Ensiled for 28 d | |||||
| pH | 5.7a | 3.1b | 5.4a | 0.10 | <0.001 |
| Lactic acid (% of DM) | 0.18b | 1.12a | 0.26b | 0.08 | <0.001 |
Table 5
Effects of the co-ensiling of garlic stalk with citrus pulp on the chemical composition after 7, 14, and 28 d of ensiling
| Items | Treatment1) | SE | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| GS 100% | CP 100% | GS 70% +CP 30% | |||
| Ensiled for 0 d | |||||
| Dry matter (%) | 61.1b | 13.7c | 63.4a | 0.31 | <0.001 |
| Crude protein | 5.4b | 9.3a | 5.5b | 0.12 | <0.001 |
| NDF | 52.1a | 24.0b | 51.5a | 2.22 | <0.001 |
| Ether extract | 1.8b | 4.2a | 2.1b | 0.30 | <0.001 |
| Crude ash | 13.4a | 4.1b | 12.8a | 0.43 | <0.001 |
| Ensiled for 7 d | |||||
| Dry matter (%) | 62.0a | 13.4b | 63.0a | 0.64 | <0.001 |
| Crude protein | 5.3b | 9.4a | 5.4b | 0.26 | <0.001 |
| NDF | 52.5a | 27.3b | 52.5a | 2.61 | <0.001 |
| Ether extract | 1.7b | 2.5a | 1.9b | 0.11 | <0.001 |
| Crude ash | 14.4a | 4.1c | 12.8b | 0.40 | <0.001 |
| Ensiled for 14 d | |||||
| Dry matter (%) | 63.0b | 13.7c | 64.8a | 0.51 | <0.001 |
| Crude protein | 5.1b | 9.1a | 5.5b | 0.23 | <0.001 |
| NDF | 56.6a | 26.7c | 43.4b | 1.04 | <0.001 |
| Ether extract | 1.7b | 3.4a | 2.1b | 0.21 | <0.001 |
| Crude ash | 12.9a | 4.0b | 12.6a | 0.32 | <0.001 |
| Ensiled for 28 d | |||||
| Dry matter (%) | 61.5b | 14.0c | 62.8a | 0.34 | <0.001 |
| Crude protein | 5.2b | 9.2a | 5.6b | 0.25 | <0.001 |
| NDF | 54.1a | 24.6c | 46.5b | 1.30 | <0.001 |
| Ether extract | 1.9b | 3.9a | 2.1b | 0.12 | <0.001 |
| Crude ash | 13.3a | 4.0b | 13.0a | 0.21 | <0.001 |
Table 6
Effects of the addition of citrus pulp to garlic stalk on the silage quality, sensory, and physical parameters after 14 d of ensiling
| Items | Treatment1) | SE | p-value | |||
|---|---|---|---|---|---|---|
|
|
||||||
| GS 70%+CP 30% | GS 60%+CP 40% | GS 50%+CP 50% | GS 40%+CP 60% | |||
| d 0 (before ensiling) | ||||||
| WSC (of DM) | 1.7 | 1.8 | 1.9 | 1.7 | 0.12 | 0.233 |
| NH3-N (ppm) | 291b | 395a | 279b | 248b | 29.1 | <0.001 |
| Ensiled for 14 d | ||||||
| WSC (% of DM) | 1.7 | 1.9 | 1.9 | 1.8 | 0.14 | 0.418 |
| NH3-N (ppm) | 444ab | 533a | 325bc | 279c | 36.7 | <0.001 |
| Lactic acid bacteria, log10 cfu/g2) | 7.3 | 7.2 | 7.7 | 7.1 | 0.20 | 0.101 |
| Sensory and physical parameters | ||||||
| Fermentation odor3) | 3.0b | 3.1b | 3.4a | 3.4a | 0.10 | <0.001 |
| Putrid odor4) | 0 | 0 | 0 | 0 | - | - |
| Garlic odor5) | 1.0a | 0.6b | 0.4c | 0.3c | 0.11 | <0.001 |
| Moldy appearance6) | 0 | 0 | 0 | 0 | - | - |
| Absorption degree7) | 3.6b | 4.0a | 4.2a | 3.4b | 0.12 | <0.001 |
Table 7
Effects of the addition of incremental proportions of citrus pulp to garlic stalk on the chemical composition after 14 d of ensiling
| Items | Treatment1) | SE | p-value | |||
|---|---|---|---|---|---|---|
|
|
||||||
| GS 70%+CP 30% | GS 60%+CP 40% | GS 50%+CP 50% | GS 40%+CP 60% | |||
| d-0 (before ensiling) | ||||||
| Dry matter (%) | 63.5a | 57.4b | 45.5c | 44.5c | 1.44 | <0.001 |
| Crude protein | 7.7ab | 7.8a | 7.5ab | 7.3b | 0.10 | 0.014 |
| NDF | 41.5b | 44.7a | 37.5c | 35.0c | 1.01 | <0.001 |
| Acid detergent fiber | 45.2b | 49.4a | 42.3c | 43.2bc | 1.04 | <0.001 |
| Ether extract | 1.4a | 1.1b | 1.1b | 1.2b | 0.12 | 0.001 |
| Crude ash | 12.6a | 10.2c | 11.1b | 11.7ab | 0.31 | <0.001 |
| NFC | 36.8b | 36.2b | 42.8a | 44.8a | 1.10 | <0.001 |
| Ensiled for 14 d | ||||||
| Dry matter (%) | 62.9a | 56.3b | 48.3c | 42.2d | 2.14 | <0.001 |
| Crude protein | 7.8b | 9.3a | 8.0b | 7.9b | 0.15 | <0.001 |
| NDF | 44.3a | 45.3a | 37.4b | 32.9c | 0.50 | <0.001 |
| Acid detergent fiber | 44.7ab | 46.9a | 43.1b | 38.8c | 1.03 | <0.001 |
| Ether extract | 1.7b | 2.0a | 2.1a | 2.1a | 0.11 | 0.004 |
| Crude ash | 12.4 | 12.6 | 12.5 | 11.8 | 0.34 | 0.167 |
| NFC | 33.8c | 30.8d | 40.0b | 45.3a | 0.63 | <0.001 |
REFERENCES
1. Faostat (Food and Agriculture Organization of the United Nations). FAO Statistical Databases [Internet]. [cited 2018 Dec 6]. Available from: http://faostat.fao.org/
2. Kamruzzaman M, Liang X, Sekiguchi N, Sano H. Effect of feeding garlic leaf on microbial nitrogen supply, kinetics of plasma phenylalanine, tyrosine and protein synthesis in sheep. Anim Sci J 2014; 85:542–8. https://doi.org/10.1111/asj.12190


3. Lee YH, Kim YI, Oh YK, Ahmadi F, Kwak WS. Yield survey and nutritional evaluation of garlic stalk for ruminant feed. J Anim Sci Technol 2017; 59:22https://doi.org/10.1186/s40781-017-0147-3




4. Kallel F, Ellouz-Chaabouni S. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: a review. Environ Prog Sustain Energy 2017; 36:1765–77. https://doi.org/10.1002/ep.12649

5. McDonald P, Henderson AR, Heron SJE. The biochemistry of silage. Marlow, Bucks, UK: Chalcombe Publications; 1991.
6. Weinberg ZG, Muck RE. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol Rev 1996; 19:53–68. https://doi.org/10.1016/0168-6445(96)00025-3


7. Ni K, Wang F, Zhu B, et al. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour Technol 2017; 238:706–15. https://doi.org/10.1016/j.biortech.2017.04.055


8. Kim JS, Lee YH, Kim YI, et al. Effect of microbial inoculant or molasses on fermentative quality and aerobic stability of sawdust-based spent mushroom substrate. Bioresour Technol 2016; 216:188–95. https://doi.org/10.1016/j.biortech.2016.05.056


9. Kim KS, Park SM, Park KJ, Kim SS, Hyun JW, Hwang RJ. Status of the citrus industry in Republic of Korea. IX International Symposium on Temperate Zone Fruits in the Tropics and Subtropics 1059. 2014. p. 51–8. https://doi.org/10.17660/ActaHortic.2014.1059.4

10. Satari B, Karimi K. Citrus processing wastes: environmental impacts, recent advances, and future perspectives in total valorization. Resour Conserv Recycl 2018; 129:153–67. https://doi.org/10.1016/j.resconrec.2017.10.032

11. Scerra V, Caparra P, Foti F, Lanza M, Priolo A. Citrus pulp and wheat straw silage as an ingredient in lamb diets: Effects on growth and carcass and meat quality. Small Rumin Res 2001; 40:51–6. https://doi.org/10.1016/S0921-4488(00)00208-X


12. Bampidis VA, Robinson PH. Citrus by-products as ruminant feeds: a review. Anim Feed Sci Technol 2006; 128:175–217. https://doi.org/10.1016/j.anifeedsci.2005.12.002

13. Gebrehanna MM, Gordon RJ, Madani A, VanderZaag AC, Wood JD. Silage effluent management: a review. J Environ Manag 2014; 143:113–22. https://doi.org/10.1016/j.jenvman.2014.04.012

14. AOAC International. Official methods of analysis. 19th ed.Arlington, VA, USA: AOAC Int; 2012.
15. Licitra G, Hernandez TM, Van Soest PJ. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim Feed Sci Technol 1996; 57:347–58. https://doi.org/10.1016/0377-8401(95)00837-3

16. Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1956; 28:350–6. https://doi.org/10.1021/ac60111a017

17. Barker SB, Summerson WH. The colorimetric determination of lactic acid in biological material. J Biol Chem 1941; 138:535–54.

18. Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem 1962; 8:130–2. https://doi.org/10.1093/clinchem/8.2.130



19. Huisden CM, Adesogan AT, Kim SC, Ososanya T. Effect of applying molasses or inoculants containing homofermentative or heterofermentative bacteria at two rates on the fermentation and aerobic stability of corn silage. J Dairy Sci 2009; 92:690–7. https://doi.org/10.3168/jds.2008-1546


20. Muck RE. Factors influencing silage quality and their implications for management. J Dairy Sci 1988; 71:2992–3002. https://doi.org/10.3168/jds.S0022-0302(88)79897-5

21. Ávila CLS, Carvalho BF, Pinto JC, Duarte WF, Schwan RF. The use of Lactobacillus species as starter cultures for enhancing the quality of sugar cane silage. J Dairy Sci 2014; 97:940–51. https://doi.org/10.3168/jds.2013-6987


22. Zhao J, Dong Z, Li J, et al. Effects of lactic acid bacteria and molasses on fermentation dynamics, structural and nonstructural carbohydrate composition and in vitro ruminal fermentation of rice straw silage. Asian-Australas J Anim Sci 2019; 32:783–91. https://doi.org/10.5713/ajas.18.0543



23. Migwi PK, Gallagher JR, Van Barneveld RJ. Effect of molasses on the fermentation quality of wheat straw and poultry litter ensiled with citrus pulp. Anim Prod Sci 2000; 40:825–9. https://doi.org/10.1071/EA99011

24. Hillion ML, Moscoviz R, Trably E, et al. Co-ensiling as a new technique for long-term storage of agro-industrial waste with low sugar content prior to anaerobic digestion. Waste Manag 2018; 71:147–55. https://doi.org/10.1016/j.wasman.2017.10.024


25. Muck R. Recent advances in silage microbiology. Agric Food Sci 2013; 22:3–15. https://doi.org/10.23986/afsci.6718

26. McAllister TA, Dunière L, Drouin P, et al. Silage review: Using molecular approaches to define the microbial ecology of silage. J Dairy Sci 2018; 101:4060–74. https://doi.org/10.3168/jds.2017-13704


27. Whiter AG, Kung L. The effect of a dry or liquid application of Lactobacillus plantarum MTD1 on the fermentation of alfalfa silage. J Dairy Sci 2001; 84:2195–202. https://doi.org/10.3168/jds.S0022-0302(01)74666-8


28. Muck RE. Dry matter level effects on alfalfa silage quality II. Fermentation products and starch hydrolysis. Trans ASAE 1990; 33:373–81. https://doi.org/10.13031/2013.31340

29. Mthiyane DMN, Nsahlai IV, Bonsi MLK. The nutritional composition, fermentation characteristics, in sacco degradation and fungal pathogen dynamics of sugarcane tops ensiled with broiler litter with or without water. Anim Feed Sci Technol 2001; 94:171–85. https://doi.org/10.1016/S0377-8401(01)00311-X

30. Sibanda S, Jingura M, Topps JH. The effect of level of inclusion of the legume Desmodium uncinatum and the use of molasses or ground maize as additives on the chemical composition of grass- and maize-legume silages. Anim Feed Sci Technol 1997; 68:295–305. https://doi.org/10.1016/S0377-8401(97)00049-7







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





