Fungal diversity characteristics
Table 1 describes the total yeast and mold counts (CFU/g) in elephant grass silage samples using the traditional method. The number of fungi was high in the control group and low in the two additive groups. Yeast and mold counts were significantly different between the control and two additive groups (p<0.05). The number of fungi was high in the control and low in the additive group. Possible reasons were that the growth of fungi was inhibited by salt, which enhanced fermentation. Sugar provided carbohydrates for LAB that increased accumulation of lactic acid, resulting in a low pH that can inhibit the growth of molds and yeasts [
15]. Decreased pH and increased lactic acid resulted in a lower number of fungi in the sugar group. Many reports have shown that counts of molds and yeasts were also lower in salt-treated or sugar-treated silage, which tended to have a higher dry matter content and lower pH compared with control silage [
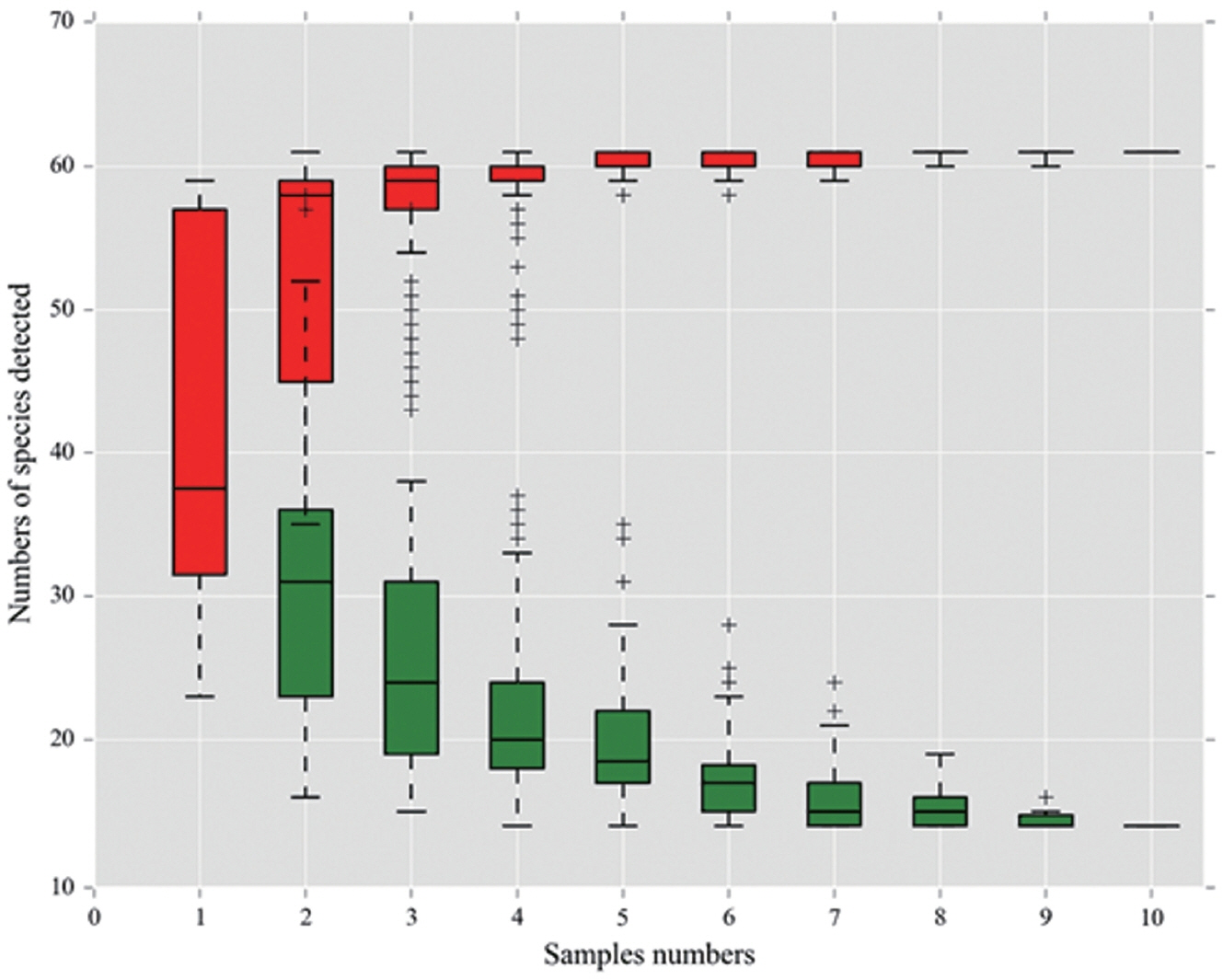
16]. Our data also showed that fungal community richness decreased when salt or salt plus sugar were added compared to the control. Theoretically, mold counts in the salt-sugar additive group should have been lower than the salt additive group. However, in this study, mold counts in the salt additive group were significantly (p<0.05) lower than salt-sugar additive group. The reason may be that the amount of sugar added (0.2% sugar on fresh material basis) may be not enough to promote epiphytic LAB activity. On the other hand, molds may utilize sugar to grow and compete with other microorganisms during aerobic fermentation. Meanwhile, to gain deeper insights into fungal community dynamics in the ensiling of elephant grass, high-throughput sequencing was used in this study. Based on the sample number and the OTUs, a species accumulation curve for all samples was calculated (
Figure 1). In this study, the curve reached a plateau and there was not an obvious increase in the number of species with an increasing number of samples, indicating that the sample number in our study was large enough to reflect species richness. The sampling completeness was evaluated by the GoodŌĆÖs coverage values which were approximately 0.99 in all samples indicating that most of the fungal community in the samples were adequately captured.
In order to identify the dynamic changes of fungi with the lengthening of fermentation time by different additives, fungal community diversity was assessed by sequencing the fungal 18S rRNA V3+V4 regions at different fermentation times with two additives (
Table 2). After removing the low-quality sequences, 2,399,340 reads with an average length of 200 bp per sequence were subjected to the following analysis. Furthermore, these reads were clustered into 216 core OTUs based on 99% similarity level (equal to the species level). The Chao1 index indicates the richness of the microbial community. Chao1 also indicates the number of species and therefore the richness of the fungal community. The larger the Chao1, the greater the number of OTUs and the greater the richness of the community contained in the group. Shannon indices, which indicate the diversity of the fungal community were negatively affected by the additives [
17]. The Chao1 index showed a sharp increase (from 157-184-200-210 at 0, 5, 15, and 60 days, respectively) in the richness of the fungal community during the elephant grass silage under natural conditions (control group). However, after adding salt or salt-sugar, the Chao1 estimator varied 157-192-88-193 and 157-177-119-136, respectively. Based on these data, we found that, after an initial increase, fungal community richness drastically decreased in the salt and salt-sugar additive groups. This result was also proved by traditional methods (
Table 1). Moreover, the salt additive group had much lower fungal community richness than the salt-sugar additive group and control. Conversely, at the end of the fermentation period the salt-sugar additive group exhibited lower fungal community richness than the salt additive group.
In addition, changes in the number of fungus OTUs during elephant grass ensiling with different additives and on different days (
Figure 2) were investigated. Fungal diversity was significant different only at genus and species levels between the additive group and the control (p<0.05), but no significant differences could be found between the two additives groups at all levels. This illustrates that salt and sugar can inhibit the growth of fungi in comparison to the control, but the differences between the two additive groups were not significant. Consistent with the number of Chao1, the total number of OTUs in the salt additive group was much less than the control and salt-sugar additive group in the middle of the fermentation process. Therefore, during this period, the number and species of fungi changed a great deal. In conclusion, the combined traditional culture method and high-throughput sequencing confirmed that adding a salt or salt-sugar mixture can effectively decrease fungal community diversity.
Heat map
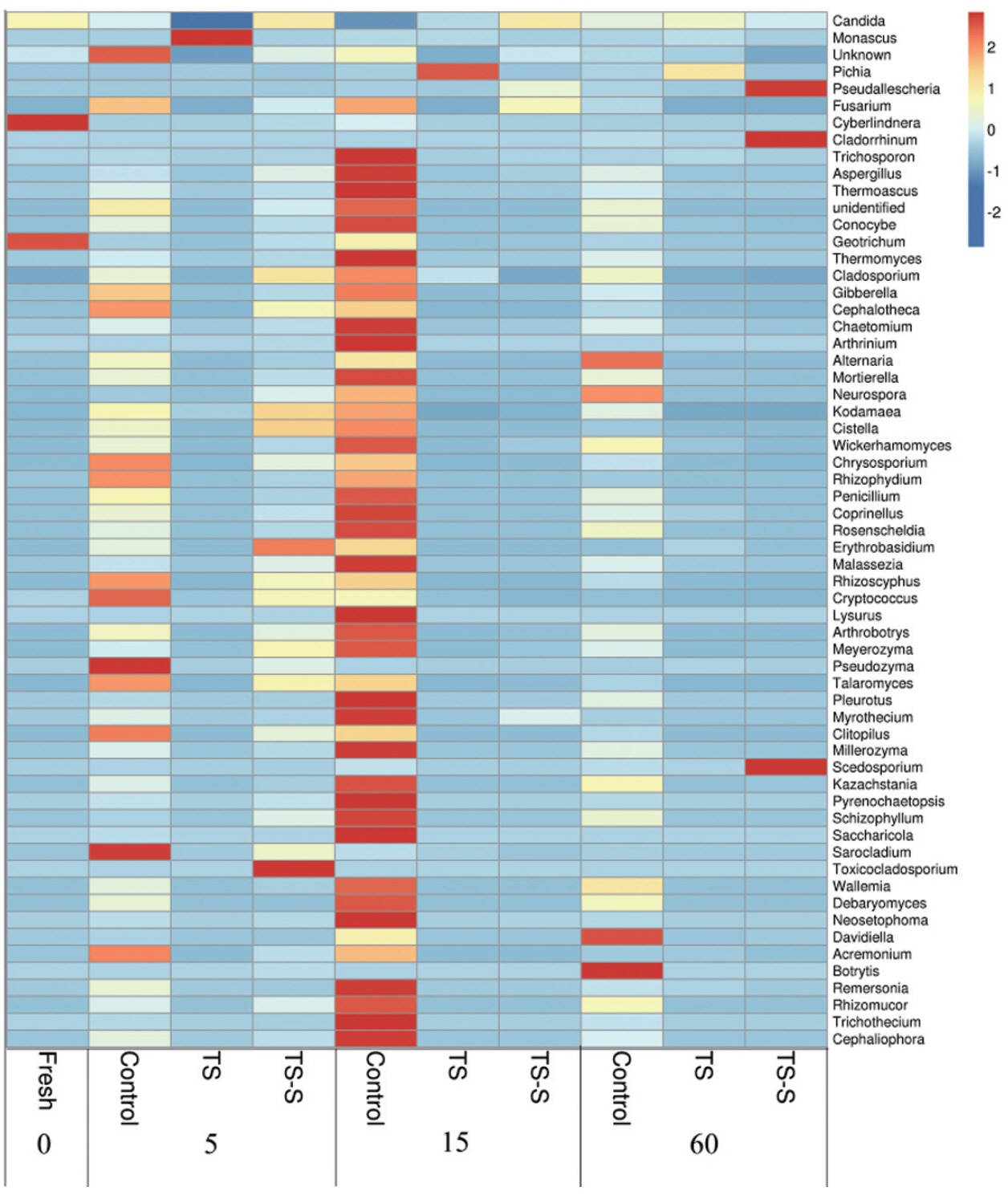
The relative abundance of fungi with the lengthening of storage time and the influence of different additives was compared by heat map analysis at genus level (
Figure 4). The deepest red color signifies high relative abundance, indicating that fungi were very active in this stage. Based on the data, the dominant fungi were molds and yeasts in all samples. Among them, four dominant phyla of molds were identified in elephant grass silage, including
Chytridiomycota,
Ascomycota,
Zygomycota, and
Basidiomycota. Small differences were shown among samples at phylum level in all groups. However, at the species level the dynamic of molds and yeasts in control were found to be different from the two additives groups. Also, a much higher diversity of fungi was found in the control (
Figure 4).
Cyberlindnera and
Geotrichum, a yeast and mold respectively, had high relative abundance in fresh elephant grass.
Geotrichum is the dominant mold in maize silage [
18]. On day 5, the predominant fungi changed to
Pseudozyma,
Sarocladium,
Cryptococcus, and
Rhizoscyphus in the control group due to initial high oxygen content and high WSC in elephant grass silage. Most of the fungi in the data were molds commonly detected in other plant silage such as maize silage [
19], and so on. Their fast growth indicates that the elephant grass had begun to rot. However, after adding salt, the relative abundance of
Pseudozyma,
Sarocladium,
Cryptococcus, and
Rhizoscyphus were very low on day 5, and few species of spoilage molds existed. The growth of spoilage molds and yeasts during the early stage would significantly affect the quality of elephant grass silage. After the addition of salt-sugar, at the beginning of the fermentation process, the growth of molds and yeasts were significantly inhibited by the salt. Therefore, the relative abundance of putrefactive organisms was more than that of the salt additive group, such as
Toxicocladosporium and
Erythrobasidium, but far less than the control group. As silage progressed, oxygen content and pH decreased. Fungi can continue to grow in these conditions. During the middle stage, on day 15, decay was worse than before in the control group, with a high relative abundance of putrefactive organisms found, including
Pseudozyma,
Fusarium, and
Penicillium. This is in contrast with the additive groups. Few red spots were found in the additive group, explaining why putrefactive organisms were not active after being treated with salt or salt-sugar. The dominant genus in the salt additive group was
Pichia due to its salt resistance [
12] and acid tolerance which can survive in salty environments. However, the overall level of spoilage molds was very low in the salt additive group, which may indicate good silage quality. Particularly at the end of fermentation, the advantage of adding salt was clear. No red spots were found in the salt-sugar additive group indicating that addition of the salt-sugar additive could inhibit the growth of putrefactive organisms effectively in the middle silage of elephant grass at the end of the fermentation period (60 days). In the salt-sugar additive group, the number of species and the relative abundance of molds and yeasts, such as
Pseudallescheria,
Cladorrhinum, and
Scedosporium, began to increase.
Cladorrhinum and
Scedosporium are infrequently associated with human and animal opportunistic infections. This may be because sugar offers the nutrients for fungus growth. However, the most common putrefactive organisms remained active in the control group, including
Botrytis and
Alternaria.
In view of the results, the diversity of the fungal community was remarkably increased during the silaging process in the control group. Moreover, the diversity and relative abundance of putrefactive organisms also maintained high levels, more so than the additive groups. This indicates that the high diversity and relative abundance of putrefactive organisms resulted in the low quality of elephant grass silage. Salt and salt-sugar treatments can significantly inhibit the growth of fungi. In particular, the addition of salt could improve the quality of elephant grass silage significantly.
Community dynamics of the predominant undesirable microorganisms
Elephant grass has been viewed as a difficult crop to ensilage, primarily because of its high water content, high water-soluble carbohydrates and low oxygen content. These conditions are conducive to the development of undesirable microorganisms, including yeasts and molds which are responsible for silage degradation with potential negative effects on animal and human health. The predominant undesirable microorganisms, including yeasts and molds, were similar across the two methods; however, more fungi were found using a high throughout method. Of the undesirable microorganisms found in silage, yeasts are the most important group as they are involved in aerobic spoilage, during the aerobic phase at the beginning of ensiling. Silage oxygenation restarts yeast organic acid metabolism pathways (succinic, citric, and lactic acids) inducing a pH increase and allowing for the growth of less acid-tolerant microorganisms. On the other hand, epiphytic yeasts present in silage can convert WSC into CO
2 and alcohols, which are known to be toxic to the liver. Alcohols can increase dry matter loss, decrease nutritional value, rapidly corrupt silage and decrease feed intake [
20].
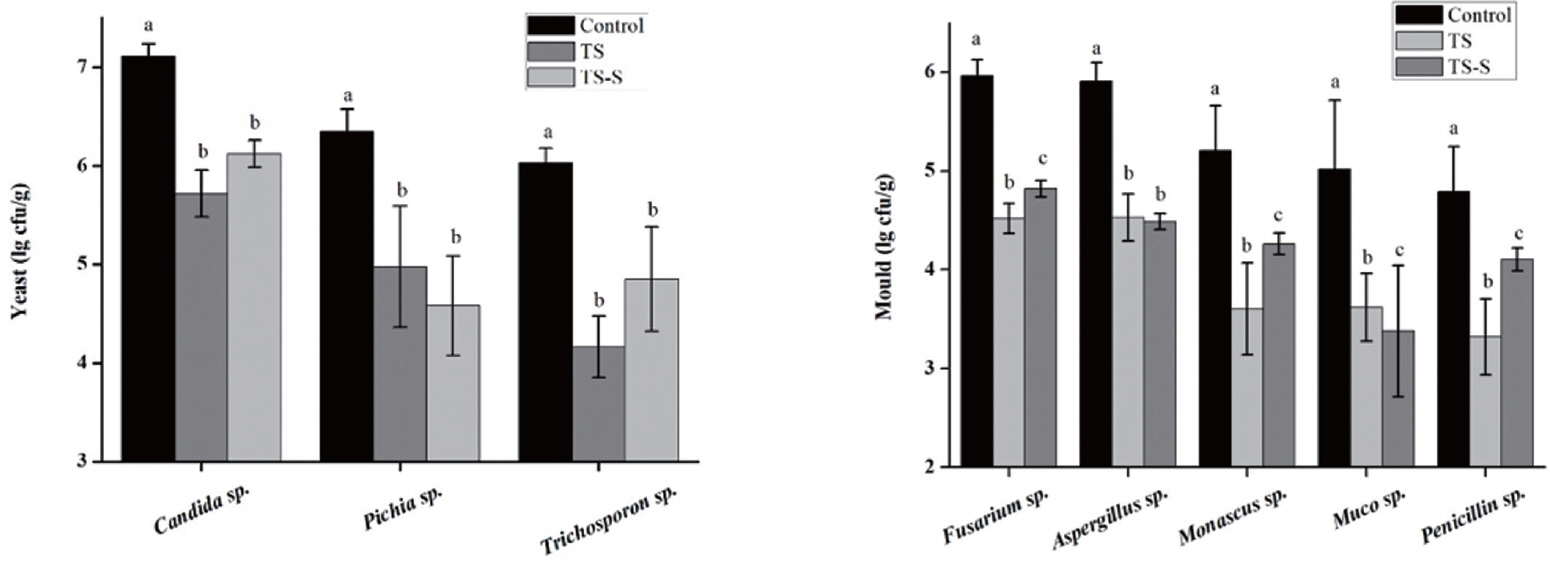
In order to improve elephant grass silage preservation and guarantee the quality of this animal feed, the species of the predominant putrefactive organisms during elephant grass fermentation were identified using traditional culture methods (
Figure 5) and high throughout methods (
Figure 6). Yeasts and molds are considered to be the most important putrefactive organisms found in silage [
21]. As shown in (
Figure 5), three kinds of yeasts and five kinds of molds were most frequently found. As expected,
Candida sp.,
Pichia sp., and
Trichosporon sp. were the top-ranked yeasts in both treatments. Furthermore, the number of each in all treatment groups was significantly different to the control group. The predominant molds identified were
Fusarium sp.,
Aspergillus sp.,
Monascus sp.,
Muco sp., and
Penicillium sp. Among them,
Fusarium sp.,
Aspergillus sp.,
Muco sp., and
Penicillium sp. were the most common undesirable molds [
22]. The number of these molds was significantly different to that seen in the control group. Differences were also present between the inter- and intra-group in the treatment group, except for
Aspergillus sp., indicating that adding salt and sugar can inhibit the growth of yeasts and molds. The diversity and relative abundance of mold after salt and sugar addition were also significantly different (p<0.05). Thus, the addition of salt and sugar could be a good approach to improve the quality of elephant grass silage.
Using the high throughout method, ten commonly oc curring OTUs at the genus level, isolated from the fungal community, were selected to identify the main spoilage fungi. The top ten fungi based on their relative abundance at the genus level are listed in (
Figure 6). This shows that the predominant fungi in elephant grass silage shifted significantly between the spontaneous fermentation and two additive groups during the fermentation period.
Candida was the predominant yeast in all groups and had a high relative abundance (over 65%). On day 0, the relative abundance of
Candida reached 83.44%. One exception was
Monascus, whose relative abundance (59.61%) far exceeded
Candida (39.13%), during the initial stage of the salt additive group. It may be that after adding salt, fermentation conditions changed so that the growth of
Candida was inhibited and
Monascus was able to quickly grow due to its resistance to heat, alcohol, lactic acid and high salt levels [
20]. Furthermore,
Monascus can survive under reduced oxygen levels when NaCl concentration levels range from 2% to 10% in the Greek table olive industries [
23]. The relative abundance of
Monascus descended rapidly when fermentation conditions became incompletely anaerobic. Overall, in the salt additive group, the relative abundance of
Candida (39.13%) was far less than the control, even lower than the salt-sugar additive group (83.44%). This can decrease the production of alcohols to reduce the risk of liver toxicity and improve silage quality. The fungi found by traditional cultivation and high-throughput analysis are slightly different, probably because some fungi cannot grow or grow slowly on culture medium thus less species of fungi were shown in traditional approach. Yeasts accounted for a large proportion in traditional method, so did in high-throughput analysis. Therefore, fewer species of molds presented among the dominant fungi species in high-throughput results.
In the early stages of fermentation, some oxygen remained for microorganism reproduction, while the respiration of plants made the type of microorganisms change dramatically. As the oxygen content declined sharply and the temperature decreased after the oxygen was used up, the available nutrients for the microorganisms gradually diminished. LAB can convert carbohydrates into carbon dioxide, ethanol and organic acids that lower the pH, which together with the anaerobic conditions, prevent the growth of spoilage organisms, such as fungi and other bacteria [
24]. However, in natural fermentation, the number of epiphytic LAB was initially very low. Along with fermentation, LAB composted insufficiently (heterofermentation) which was not enough to reduce pH value and suppress undesirable microorganisms [
25]. On day 0, the most abundant fungi were associated with the
Cyberlindnera (13.28%) and the
Fusarium (0.17%). Moreover,
Aspergillus accounted for as little as 0.01% in the beginning. On day 5,
Fusarium sp. was the most frequent spoilage mold in elephant grass silage, followed by
Aspergillus sp.,
Penicillium sp.,
Trichosporon sp., and
Cladosporium [
22], all of which are mycotoxigenic fungi [
26]. In the control group on day 15, the most abundant spoilage molds were
Fusarium sp. (5.47%),
Trichosporon sp. (5.22%), and
Aspergillus sp. (3.68%). Under these conditions, spoilage molds reproduce quickly and gradually deteriorate the elephant grass. Therefore, natural fermentation silage resulted in unsatisfactory preservation due to the relatively low content of lactic, acetic and butyric acids, the high pH, and the promotion in the breakdown of protein to NH
3-N [
27]. Based on this, it would be necessary to add LAB with the salt and sugar to further improve the silage quality of elephant grass.
Additives can have significant effects on the quality of si lage by enabling the production of more organic acids and greater reductions in pH, as well as effectively inhibiting undesirable microorganisms [
11,
28]. Compared to the control group, the fungal community changed after the addition of salt or salt sugar. In the salt-sugar group,
Pseudallescher was dominant in the middle and later stages, which was linked to a decrease in the protein content and nutritional value of the silage for the production of biogenic amines, including putrescine, cadaverine and tyramine in silage [
29].
Fusarium and
Aspergillus always showed the lowest relative abundance and could not be detected at the end of the fermentation period. Few spoilage molds were found in the salt additive group. Overall, using additives modified the structure of fungi and reduced the diversity and relative abundance of undesirable microorganisms compared to the control group.
In theory, the relative abundance of molds and yeasts in the salt-sugar additive group should decrease, in contrast to the control and salt additive groups. While salt acts to sterilize, sugar can increase the water soluble carbohydrate content which is converted into organic acids, mainly lactic acid by LAB, thereby reducing pH. It has been suggested that salt and sugar addition could effectively inhibit the growth of harmful spoilage fungi and improve the silage quality of elephant grass. However, in this study, undesirable fungi in the salt additive group were much less than that found in the salt-sugar additive group. There are two primary reasons for this result: i) poor sealing in the salt-sugar additive group prolonged oxygen consumption; and ii) molds and LAB in the salt-sugar additive group grew competitively for sugar. One study showed that with the addition of 3% molasses, dwarf elephant grass had a higher content of lactic acid, dry matter and crude protein and a lower pH compared with the control perhaps 0.2% sugar added to elephant grass silage may be not enough.
In conclusion, the addition of 0.5% salt and 0.5% saltŌłÆ0.2% sugar can effectively improve the silage quality of elephant grass. However, the total fungal counts in this study were above 1├Ś10
4 CFU/g, which exceeded the quality standard for animal feeds [
30] (
Table 1). In
Table 1, the total counts of yeasts and molds present in the salt and salt-sugar additive groups were 7.9├Ś10
5, 8.7├Ś10
4, 1.9├Ś10
6, and 1.7├Ś10
5, respectively. Hence, more research is needed to further decrease the number of fungi in silage. Recently, many papers reported that the addition of LAB could effectively improve the silage quality of barley silage [
17] and maize [
21] and reduce the abundance of undesirable microorganisms. It is necessary to use LAB and salt as additives to further improve the silage quality of elephant grass in the next stage of investigation.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





