 |
 |
| Anim Biosci > Volume 30(11); 2017 > Article |
|
Abstract
Objective
The objective of this study was to optimize ultrasonic-assisted enzymatic hydrolysis conditions, including enzyme-to-substrate (E/S) ratio, pH, and temperature, for producing porcine liver hydrolysates (PLHs) with the highest 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity by using response surface methodology (RSM).
Methods
The study used RSM to determine the combination of hydrolysis parameters that maximized the antioxidant activity of our PLHs. Temperature (40┬░C, 54┬░C, and 68┬░C), pH (8.5, 9.5, and 10.5), and E/S ratio (0.1%, 2.1%, and 4.1%) were selected as the independent variables and analyzed according to the preliminary experiment results, whereas DPPH free radical scavenging activity was selected as the dependent variable.
Results
Analysis of variance showed that E/S ratio, pH, and temperature significantly affected the hydrolysis process (p<0.01). The optimal conditions for producing PLHs with the highest scavenging activity were as follows: E/S ratio, 1.4% (v/w); temperature, 55.5┬░C; and initial pH, 10.15. Under these conditions, the degree of hydrolysis, DPPH free radical scavenging activity, ferrous ion chelating ability, and reducing power of PLHs were 24.12%, 79%, 98.18%, and 0.601 absorbance unit, respectively. The molecular weight of most PLHs produced under these optimal conditions was less than 5,400 Da and contained 45.7% hydrophobic amino acids.
Porcine liver, a byproduct of pig slaughtering, contains many nutrients [1]; however, it is rarely consumed probably because of its high cholesterol content [2]. Underutilization of such byproducts leads to potential economic loss and increases disposal cost. Enzymatic hydrolysis is used to recover and improve the nutritional and functional properties of proteins from fish and animal byproducts, such as tuna [3,4], chicken [5], and porcine liver [1], to yield valuable components. Alcalase, an endoproteinase, can hydrolyze proteins to peptides possessing hydrophobic amino acids at the end of their peptide chains. These hydrophobic amino acids increase the affinity between the peptides and fatty acids [6ŌĆō8], thus attracting the antioxidant amino acid residues toward the radical species and unsaturated fatty acids, finally increasing the antioxidant activities of the hydrolysates [9,10].
Ultrasonication involves the propagation of longitudinal pressure waves, further leading to the cavitation phenomenon. Within the regions undergoing cavitation pressure changes, mechanical shear forces cause cell damage, resulting in the release of more proteins [11]. Furthermore, during ultrasonication, larger protein molecules break down into smaller particles and protein aggregate structures loosen [12]. These processes improve the efficiency of protein extraction and hydrolysis. Response surface methodology (RSM) has been widely applied to optimize experimental conditions and maximize response within a specified range of factors [13]. Some process parameters, such as type of hydrolyzing enzyme, substrate protein, degree of hydrolysis (DH), substrate pretreatment, and environmental conditions are considered as the key points to a successful hydrolysis [14]. Kristinsson and Rasco [15] indicated that enzyme reaction kinetics was remarkably affected by hydrolysis temperature and pH. The cleavage degree of protein in the substrate could be controlled and the hydrolysates with different molecular profiles and functional properties could be produced by using appropriate enzyme-to-substrate (E/S) ratios. Guerard et al [16] reported that during hydrolysis of shrimp processing discards, pH and temperature played critical roles in enhancing antiradical activity. When maintaining the hydrolysis temperature at approximately 52┬░C, it might unfold protein, expose the hydrophobic residues buried inside the molecular, facilitate the protein degradation, and eventually increase the antioxidant activity of grass carp sarcoplasmic protein hydrolysates [10]. Plant proteins are generally used as raw materials for ultrasonic-assisted enzymatic hydrolysis [8,17]. This study aimed to i) model the effects of E/S ratio, pH, and temperature on the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity and DH of the ultrasonic-assisted enzymatic hydrolysis of porcine liver protein, ii) optimize the reaction to obtain the maximum DPPH free radical scavenging activity by using RSM, and iii) characterize the porcine liver protein hydrolysates (PLHs) obtained under the optimal conditions.
PLHs were produced according to the methods of Hsu [18] with some modifications. Porcine liver (50 g) was homogenized (NISSEI AM-12, Tokyo, Japan) with 100 mL of distilled water at 10,000 rpm for 1 min and heated at 95┬░C to 100┬░C for 5 min to inactivate indigenous enzymes. The homogenized solution was then equilibrated to a hydrolysis temperature (40┬░C, 54┬░C, and 68┬░C) with the pH was adjusted to 8.5, 9.5, and 10.5 by using 1.0 N NaOH, after which Alcalase 2.4L (Sigma Chemical Co., St. Louis, MO, USA) was added (0.1%, 2.1%, and 4.1% of E/S ratio, v/w) and the solution was further hydrolyzed for 30 min in an ultrasonic cleaner (40 kHz, 400 W; DELTA DC400H, New Taipei City, Taiwan). After the reaction, the mixture was heated at 95┬░C to 100┬░C for 10 min to inactivate Alcalase and then centrifuged (Himac centrifuge SCR20B, Hitachi, Tokyo, Japan) at 10,000 g for 10 min. The supernatant was filtered through a Whatman No. 1 filter paper to obtain PLHs. After the DH was determined, PLHs were lyophilized and stored at ŌłÆ20┬░C until further analysis.
We used RSM to determine the combination of hydrolysis parameters that maximized the antioxidant activity of our PLHs. Temperature, pH, and E/S ratio were selected as the independent variables and analyzed according to the preliminary experiment results, whereas DPPH free radical scavenging activity was selected as the dependent variable. A BoxŌĆōBehnken design comprising 15 experiments was used. The experiment design and dependent variable values are presented in Table 1.
Regression analysis was performed for the experiment data and fitted into the following second-order polynomial model:
Where Y is the dependent variable (i.e., DPPH free radical scavenging activity); X1, X2, and X3 are independent variables [19]; ╬▓0 is a constant term; ╬▓1, ╬▓2, and ╬▓3 are the linear, quadratic, and cross-product regression coefficients. The analysis of experimental design, calculation of predicted data, and plotting of surface plot were conducted using Minitab 16 (Minitab Inc., State College, PA, USA).
The DHs of PLHs were determined by measuring the increase in free amino groups by using a picrylsulfonic acid solution according to the method of Adler-Nissen [20] and calculated as follows:
Where htot is the total number of peptide bonds per protein equivalent, and h is the number of hydrolyzed bonds. The htot factor is dependent on raw material amino acid composition.
The ferrous ion chelating ability of PLHs was determined using the method of Dinis et al [21] and calculated as follows:
The molecular weight distribution of PLHs was determined according to the methods of Aspmo et al [24] with some modifications. Samples (1 ╬╝L) were analyzed using matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS; Voyager DE-PRO, Applied Biosystems, NY, USA) in a positive ion mode, whereas the results were obtained in the linear mode. The amino acid composition of the freeze-dried PLHs was determined using a modified method of You and Wu [25]. Briefly, 0.1 g of PLHs was hydrolyzed with 3 mL of 4 M methanesulfonic acid, containing 0.2% 3-(2-aminoethyl) indole under vacuum at 115┬░C for 48 h. The total amino acids were analyzed using an Agilent 1100 series high performance liquid chromatography system (Agilent 1100 Series HPLC Value System, Agilent Technologies, Santa Clara, CA, USA) equipped with ZORBAX Eclipse-AAA column (4.6├Ś150 mm, 3.5 ╬╝m).
The optimization experiments were conducted using RSM with the experimental design model of Minitab 16. The software generated equations describing the behavior of the hydrolysis conditions and antioxidant activities and their coefficient of determination (R2). Significance was determined at a 95% probability level.
E/S ratio, pH, and temperature are the crucial parameters affecting enzyme efficiency during protein hydrolysis [13,16,19]. Here, the ranges of optimal E/S ratio, pH, and temperature were 0.1% to 4.1%, 8.5 to 10.5, and 40┬░C to 68┬░C, respectively (Table 1). The resulting DPPH radical scavenging activities and DH values were 66.3% to 79.7%, and 16.9% to 26.9%, respectively.
A response surface model was plotted; the regression coefficients for the linear regression, quadratic regression, and inter-action of the three variables (X1, X2, and X3) on the response values (Y) are presented in Table 2. All three independent variables, E/S ratio, pH, and temperature, demonstrated the highest significant effect on the DPPH radical scavenging activity within a 99% confidence interval (p = 0.001, 0.000, and 0.007 for X1, X2, and X3, respectively). All quadratic terms X1X1 (p<0.009), X2X2 (p<0.005), and X3X3 (p<0.001) as well as the interaction term X1X3 (p = 0.05) were significant. The results indicated the quadratic items and interactions have great effects on the dependent valuables. Comparison of the monomial coefficient of the regression equation provided the following list of relevant factors in the order of the influence on the DPPH free radical scavenging activity of PLHs: X2 (initial pH)>X1 (E/S ratio)>X3 (temperature). By using RSM, Fang et al [26] indicated that E/S ratio and hydrolysis temperature and time significantly influenced the DPPH radical scavenging activity of hydrolysates derived from flying squid muscle protein. Yu et al [8] reported that the initial pH, ultrasonic power, and reaction temperature significantly influenced the DPPH free radical scavenging activity of peanut hydrolysates.
All terms with statistical significances below 5% probability level were eliminated (Table 2), providing a simplified model:
Because the values of the linear coefficients for E/S ratio (X1) were negative, a decrease in X1 increased the value of Y (i.e., DPPH radical scavenging activity). The variance analysis of the model given in Table 2 shows that the p value for the model was 0.001, indicating that the model can adequately monitor the optimization [10]. The R2 value (0.9809) was close to 1, and the adjusted R2 value was 0.9465, indicating that the model explained 94.65% of the variation in the data and that the experiment error was small; both the R2 values were highly significantly, indicating represented the actual relationships between the reaction parameters [10]. The lack-of-fit value of 0.407 indicated that the lack-of-fit is nonsignificantly associated with the pure error, illustrating that the selected model was adequate at a 95% confidence level to describe the observed data [26].
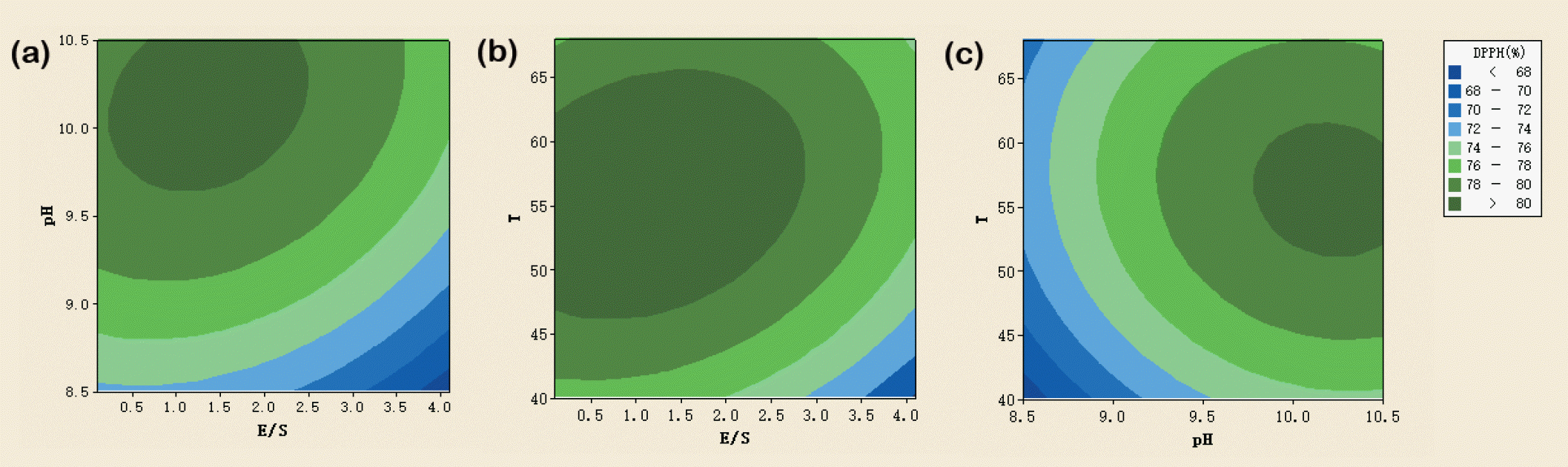
In this study, the effect of each pair of independent variables on the DPPH free radical scavenging activity of PLHs was shown using contour plots. Figure 1a indicates that with the temperature fixed at 54┬░C, the maximum scavenging activity was more than 80% when the E/S ratio was 0.2% to 2.5% and that the pH was above approximately 9.7. When the pH was fixed at 9.5, the maximum scavenging activity was obtained at a temperature of 46┬░C to 65┬░C and the E/S ratio of less than 2.8% (Figure 1b). Finally, at a fixed E/S ratio of 2.1%, the maximum scavenging activity was observed at a temperature of 52┬░C to 62┬░C and a pH of more than 9.8 (Figure 1c). The antioxidant activity was the highest at high pH, low E/S ratio, and moderate temperature. Chabeaud et al [19] reported that appropriate hydrolysis temperature and pH enhance the production of antioxidant peptides, subsequently increasing the antioxidant activity of the hydrolysates. During hydrolysis, Sarmadi and Ismail [7] clarified that proteolysis unfolds protein chains, thus exposing more active amino acid R groups and increasing the antioxidant activity.
The effects of reaction temperature on the antioxidant activity have been well-documented. Generally, the reaction rates increase with temperature, particularly in the approximate range of 40┬░C to 60┬░C (observed in the current study); this is consistent with a study by Kamau and Lu [14], who indicated that more hydrolyzed products are produced during the hydrolysis. However, when the temperature surpasses a certain level, (68┬░C in this study), the denatured enzymes and substrates may counteract its antioxidant activity because of the excessively high temperatures [8]. As biological catalysts, catalytic activities of proteases are intensively affected by pH. Kamau and Lu [14] reported that changes in pH may affect the charge distribution and conformation of the molecules, possibly disturbing the ionic character of the substrate and denaturing the protein structures of the enzyme, thus affecting the binding ability between the substrate and enzyme. Yu et al [8] clarified that more active sites became exposed in a certain pH range after the spatial structures of protease changed. Some antioxidant hydrolysates are generated when the peptide bonds in the substrate protein are broken, thus increasing the DPPH free radical scavenging activity. However, as the temperature deviates from the optimal range, the protease denatures, which reduces its scavenging activity. Figure 1c indicated that the optimal pH for Alcalase activity was approximately 9.7 to 10.5, corroborating the results of previous studies [16]. When the substrate mass fraction was maintained constant and enzyme dosage was low, several substrate molecules bound to the protease active sites and formed inactive intermediate products; therefore, the antioxidant activity either remained constant or increased gradually with enzyme dosage [8]. As the enzyme concentration increased, proteases bound to more substrate molecules, liberating more antioxidant peptides and increasing antioxidant activities. However, when the enzyme dosage increased to some extent, the excessive hydrolysis probably reduced the probability of contact between the protease and substrate molecules, finally reducing the antioxidant activity of the hydrolysates [27].
In this study, we observed that even at lower E/S ratios, such as 0.1% (Table 1) and less than 0.5% (Figure 1a, 1b); the hydrolysates showed favorable DPPH free radical scavenging activities. Yu et al [8] reported that during ultrasonic-assisted enzymatic hydrolysis, some bubbles produced through cavitation burst instantaneously, causing extensive mechanical shearing action to loosen substrate protein structures and benefit the enzymatic reaction. In this study, Alcalase easily attacked the inner protein regions, particularly the protein structures that loosened and were degraded because of ultrasonication; this resulted in the release of the hydrophobic amino acids and eventually increased the antioxidant activity [28]. As shown in Figure 1a, even at a lower E/S ratio (i.e., <0.5%) and pH of approximately 9.7 to 10.5, the PLH showed high antioxidant activity. Guerard et al [16] reported that at a high pH of 9.7, chemical hydrolysis may contribute to the hydrolysis process even without adding Alcalase. This may also clarify the high antioxidant activities at higher pH values and lower E/S ratios observed in the current study.
Here, the following optimal conditions for ultrasonic-assisted enzymatic hydrolysis for the preparation of PLHs were determined using a typical analysis of an experimental model: E/S ratio (X1), 1.4333%; initial pH (X2), 10.1566; and reaction temperature (X3), 55.6┬░C; the resulting DPPH free radical scavenging activity was predicted to be 80.73%. To confirm the validity of the predicted model, a verification test was performed using the modified process parameters including E/S ratio (X1) of 1.4%, initial pH (X2) of 10.15, and reaction temperature (X3) of 55.5┬░C under practical considerations. The verification test results illustrated a scavenging activity of 79%, close to the predicted value (80.73%); the results confirmed the appropriateness of the model for estimating experimental values.
In the present study, the DH, DPPH radical scavenging activity, reducing power, and ferrous ion chelating ability of PLHs produced under the optimal conditions were 24.12%, 79%, 0.601 absorbance unit, and 98.18%, respectively. Yu et al [8] and Fang et al [26] reported that hydrolysates obtained from Alcalase exhibited superior antioxidant activities (such as DPPH free radical scavenging activity, reduction capacity and metal ion chelation). Some free metal ions (e.g., Fe2+, Cu+) can catalyze H2O2 to hydroxyl radical in the Fenton reaction. Our PLHs could probably donate hydrogen atoms and bind ferrous ions, thus reduced ferrous ion concentrations and accelerated the H2O2 to H2O conversion, and eventually enhanced antioxidant functions [8].
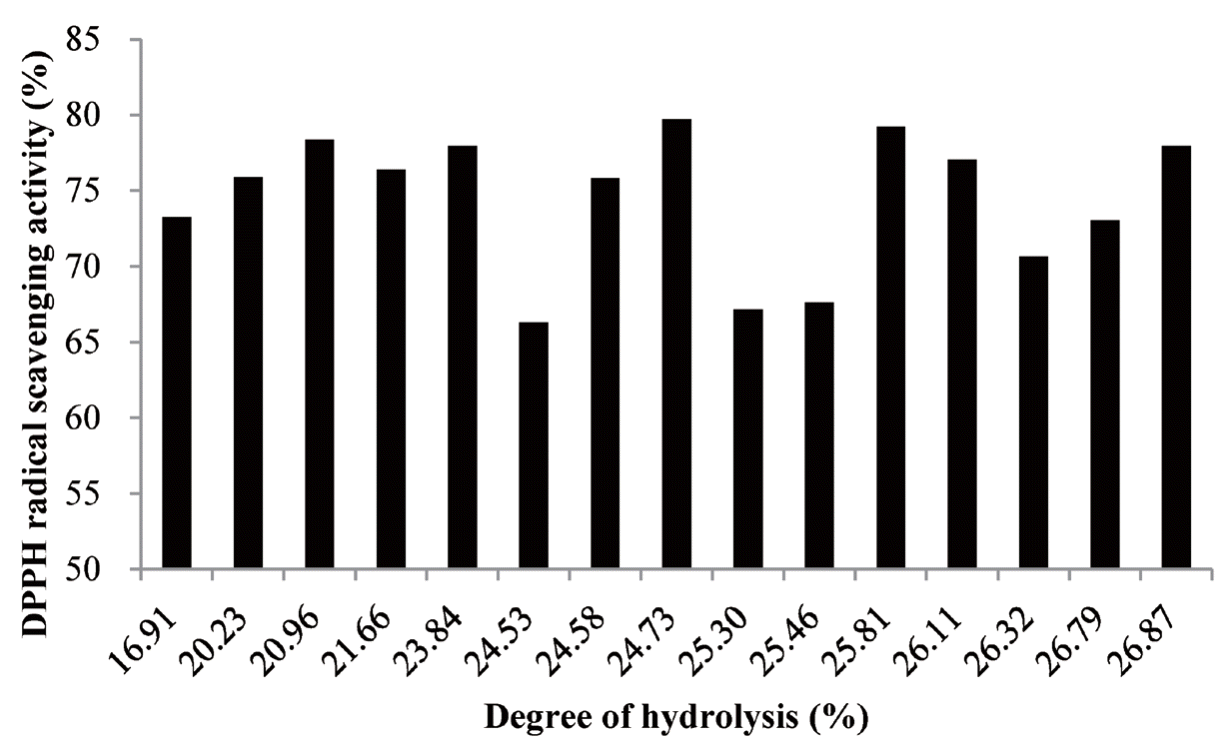
The dispersion of the points for the graphical representation of the relationship between the DPPH scavenging activity and DH can be noted in Figure 2. The results demonstrated that the scavenging activities of PLHs fluctuated with the changes in the DH values. No certain pattern or relationship was observed between the two variables in the current study, which corroborates the results of Wu et al [29] and Chabeaud et al [19], who reported that DH did not appear to be the major criterion in the production of hydrolysate with antioxidant properties. DH, defined as the percentage of peptide bonds cleaved by protease, has been frequently applied as the index of DH [19,20]. During the increased protein hydrolysis, many low molecular weight peptides and free amino acids with a concomitant increase in the numbers of ionizable groups (NH3+ and COOŌłÆ) were generated. In the meanwhile, a variation of the molecular structure caused the buried hydrophobic interior to be exposed. The properties of peptides such as molecular structure and size, net charge, and hydrophobicity were also altered [15]. It might enhance the antioxidant activity of hydrolysates. However, during the enzymatic hydrolysis, antioxidant peptides could be formed and degraded continuously. It was primarily influenced by hydrolysis conditions and further changed their molecular structure and sequence. Therefore, the DPPH scavenging activity exhibited alternating increases and decreases during the process of hydrolysis [19]. Hydrolyzed proteins with lower molecular weights (i.e., <5 kDa) have been shown to display high antioxidant activities [9,10]. In theory, hydrolysates with high DH containing more low-molecular-weight peptides may have higher antioxidant properties. However, Hsu [18] observed that peptides with low molecular weight (i.e., 390 to 1,400 Da) exhibited higher antioxidant activity than did those with a molecular weight of <390 Da, probably because some antioxidant peptides may lose their functional properties after extensive hydrolysis.
Many factors, such as operational conditions, protease type, DH, specific hydrolysate amino acid sequence, peptide structure, and peptide concentration, affect the antioxidant activity of hydrolysates. Therefore, Sarmadi and Ismail [7] reported that the overall antioxidant activity of hydrolysates should be ascribed to the integrative effects of these factors rather than to the individual actions of peptides or amino acids; moreover, the authors indicated that enzyme specificity affects the amount and composition of free amino acid and peptides as well as their amino acid sequence, subsequently influencing the molecular size, hydrophobicity, and antioxidant activity of the hydrolysates.
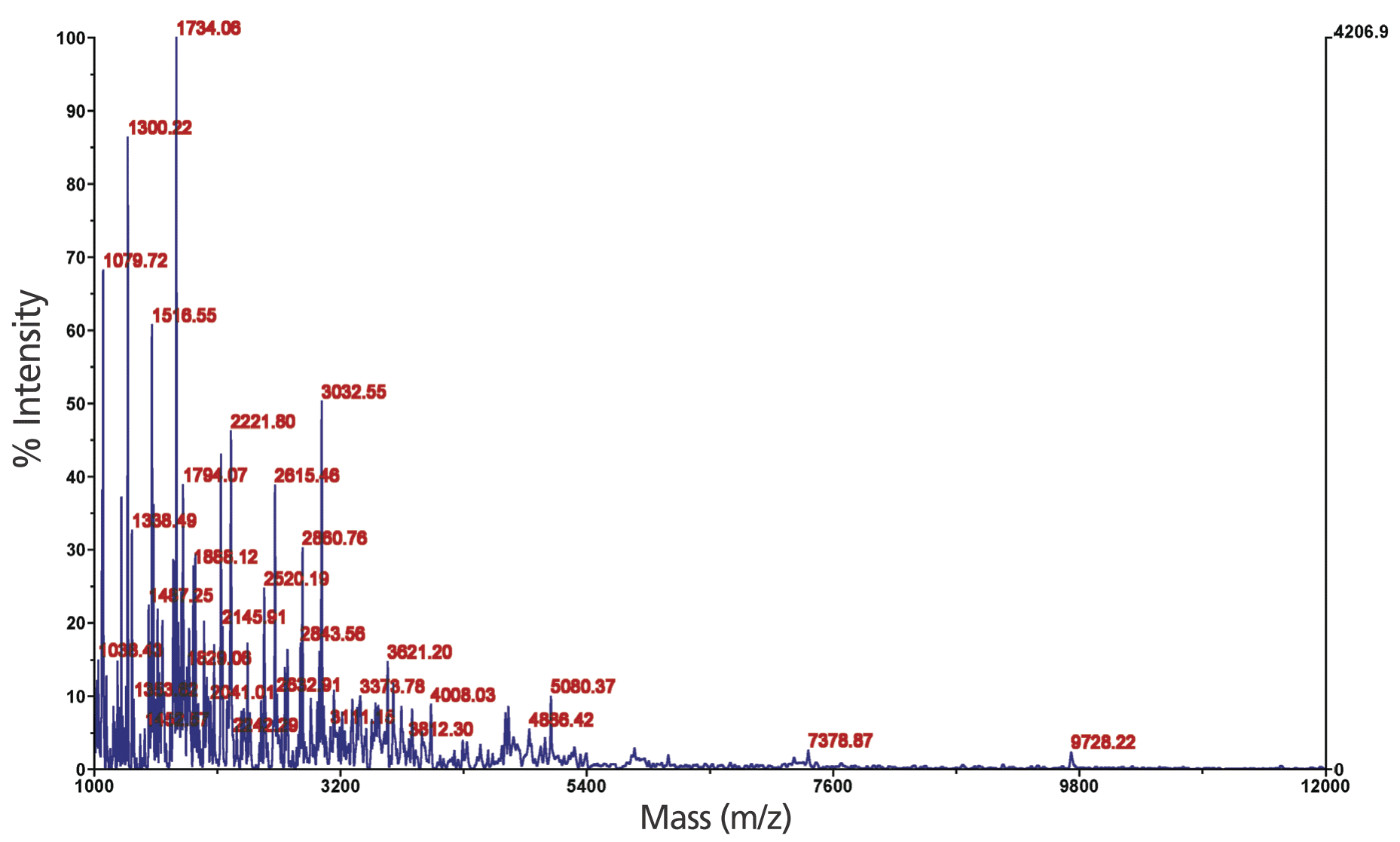
The molecular weight distribution of PLHs was determined through matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS). According to the spectrum shown in Figure 3, most of the molecular weight distribution of the PLHs produced under the optimal conditions was less than 5,400 Da, with several antioxidant properties similar to those of other hydrolysates with a molecular weight less than 5,000 Da [9,27,29].
Table 3 lists that the percentages of the hydrophobic amino acids and aromatic amino acids in the PLHs produced under optimal conditionsŌĆö47.50% and 10.88%, respectively. Hydrophobic amino acids (i.e., Gly, Ala, Val, Trp, Phe, Ile, Leu, Pro, and Met) improve the solubility of the peptides in lipids, thus promoting the peptides better interaction with hydrophobic radical species and hydrophobic polyunsaturated fatty acids [30]. By contrast, aromatic amino acids can donate protons to electron deficient radicals, stabilizing them; moreover, they can maintain the molecular stability through a resonance structure and then enhance the radical scavenging properties of the amino acids residues [7]. Another contributor to the increased antioxidant properties of the PLHs could be the high content of Asp (10.65%), considered a metal ion chelator and a hydrogen donor because it has carboxyl and amino groups in the side chain [7].
The DPPH free radical scavenging activity of our PLHs reached 79% under the optimal conditions, which were as follows: E/S ratio, 1.4%; reaction temperature, 55.5┬░C; and initial pH, 10.15. The PLHs showed the highest antioxidant activities and DH and high content of essential amino acids and hydrophobic amino acids. The MALDI-TOF MS spectra showed that the molecular weight of most hydrolysates was less than 5,400 Da. In conclusion, ultrasonic-assisted enzymatic hydrolysis can be applied to obtain favorable antioxidant hydrolysates from porcine liver with potential applications in food products for preventing lipid oxidation.
Figure┬Ā1
Contour plots of 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity as functions of (a) enzyme-to-substrate (E/S) ratio vs pH at temperature of 54┬░C, (b) E/S ratio vs temperature at pH of 9.5, and (c) pH vs temperature at an E/S ratio of 2.1%.
Figure┬Ā2
Relationship between 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and degree of hydrolysis (DH) of porcine liver protein hydrolysates.
Figure┬Ā3
Molecular weight distribution of porcine liver protein hydrolysates produced under the optimal conditions assessed through matrix-assisted laser desorption ionization-time-of-flight mass spectrometry.
Table┬Ā1
BoxŌĆōBehnken design matrix and responses of dependent variables for antioxidant hydrolysate produced from porcine liver protein
Table┬Ā2
Estimate-coded regression coefficients for 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of porcine liver protein hydrolysates
Table┬Ā3
Amino acid compositions of porcine liver protein hydrolysates produced under the optimal conditions
| Amino acid | Content (g/100 g of PLH) |
|---|---|
| Asp | 5.97 |
| Glu | 8.12 |
| Cys | 1.05 |
| Ser | 2.05 |
| His | 1.56 |
| Gly | 3.59 |
| Thr | 2.20 |
| Arg | 2.36 |
| Ala | 4.01 |
| Tyr | 1.34 |
| Val | 3.96 |
| Met | 1.31 |
| Trp | 0.42 |
| Phe | 2.78 |
| Ile | 2.78 |
| Leu | 5.50 |
| Lys | 4.78 |
| Pro | 2.30 |
| TAA | 56.1 |
| THAA1)/TAA% | 47.50 |
| TAAA2)/TAA% | 10.88 |
REFERENCES
1. Shimizu M, Tanabe S, Morimatsu F, et al. Consumption of pork-liver protein hydrolysate reduces body fat in otsuka long-evanstokushima fatty rats by suppressing hepatic lipogenesis. Biosci Biotech Bioch 2006; 70:112ŌĆō8.

2. Ockerman HW, Basu L. By-products/edible, for human consumption. Jensen WK, editorEncyclopedia of meat sciences. Amsterdam, Netherlands: Elsevier Ltd; 2004. p. 104ŌĆō12.

3. Je YJ, Lee KH, Lee MH, Ahn CB. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res Int 2009; 42:1266ŌĆō72.

4. Ahn CB, Lee KH, Je JY. Enzymatic production of bioactive protein hydrolysates from tuna liver: effects of enzymes and molecular weight on bioactivity. Int J Food Sci Technol 2010; 45:562ŌĆō8.

5. Yang KT, Lin C, Liu CW, Chen YC. Effects of chicken-liver hydrolysates on lipid metabolism in a high-fat diet. Food Chem 2014; 160:148ŌĆō56.


6. Mendis E, Rajapakse N, Byun HG, Kim SK. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci 2005; 77:2166ŌĆō78.


7. Sarmadi BH, Ismail A. Antioxidative peptides from food proteins. A review. Peptides 2010; 31:1949ŌĆō56.


8. Yu L, Sun J, Liu S, et al. Ultrasonic-assisted enzymolysis to improve the antioxidant activities of peanut (Arachin conarachin L.) antioxidant hydrolysate. Int J Mol Sci 2012; 13:9051ŌĆō68.



9. Dong S, Zeng M, Wang D, et al. Antioxidant and biochemical properties of protein hydrolysates prepared from Silver carp (Hypophthalmichthys molitrix). Food Chem 2008; 107:1485ŌĆō93.

10. Ren J, Zhao M, Shi J, et al. Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT-Food Sci Technol 2008; 41:1624ŌĆō32.

11. Iida Y, Tuziuti T, Yasui K, Kozuka T, Towata A. Protein release from yeast cells as an evaluation method of physical effects in ultrasonic field. Ultrason Sonochem 2008; 15:995ŌĆō1000.


12. Jambrak AR, Lelas V, Mason TJ, Kre┼Īi─ć G, Badanjak M. Physical properties of ultrasound treated soy proteins. J Food Eng 2009; 93:386ŌĆō93.

13. Ovissipour M, Kenari AA, Motamedzadegan A, Nazari RM. Optimization of enzymatic hydrolysis of visceral waste proteins of yellowfin tuna (Thunnus albacares). Food Bioproc Tech 2012; 5:696ŌĆō705.

14. Kamau SM, Lu RR. The effect of enzymes and hydrolysis conditions on degree of hydrolysis and DPPH radical scavenging activity of whey protein hydrolysates. Curr Res Dairy Sci 2011; 3:25ŌĆō35.

15. Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Cr Rev Food Sci 2000; 40:43ŌĆō81.

16. Guerard F, Sumaya-Martinez MT, Laroque D, Chabeaud A, Dufoss├® L. Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem 2007; 42:1486ŌĆō91.

17. Zhu KX, Su CY, Guo XN, Peng W, Zhou HM. Influence of ultrasound during wheat gluten hydrolysis on the antioxidant activities of the resulting hydrolysate. Int J Food Sci Technol 2011; 46:1053ŌĆō9.

18. Hsu KC. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem 2010; 122:42ŌĆō8.

19. Chabeaud A, Dutourni├® P, Gu├®rard F, Vandanjon L, Bourseau P. Application of response surface methodology to optimise the antioxidant activity of a saithe (Pollachius virens) hydrolysate. Mar Biotechnol 2009; 11:445ŌĆō55.

20. Adler-Nissen J. A review of food protein hydrolysis specific areas: enzymic hydrolysis of food proteins. New York, USA: Elsevier Applied Science Publications; 1986. p. 57ŌĆō131.
21. Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994; 315:161ŌĆō9.


22. Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr 1986; 44:307ŌĆō15.

23. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the anti-oxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 1992; 40:945ŌĆō8.

24. Aspmo SI, Horn SJ, Eijsink VGH. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem 2005; 40:1957ŌĆō66.

25. You SJ, Wu J. Angiotensin-I converting enzyme inhibitory and antioxidant activities of egg protein hydrolysates produced with gastrointestinal and nongastrointestinal enzymes. J Food Sci 2011; 76:C801ŌĆōC7.


26. Fang X, Xie N, Chen X, Yu H, Chen J. Optimization of antioxidant hydrolysate production from flying squid muscle protein using response surface methodology. Food Bioprod Process 2012; 90:676ŌĆō82.

27. Zhuang YL, Zhao X, Li BF. Optimization of antioxidant activity by response surface methodology in hydrolysates of jellyfish (Rhopilema esculentum) umbrella collagen. J Zhejiang Univ Sci B 2009; 10:572ŌĆō9.



28. Jia J, Ma H, Zhao W, et al. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem 2010; 119:336ŌĆō42.

- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





