 |
 |
| Anim Biosci > Volume 28(6); 2015 > Article |
|
Abstract
Lactobacillus plantarum is used for fermentation of fish products, meat and milk. However, the utilization of these bacteria in egg processing has not been done. This study was designed to evaluate the potential of fermented egg albumen as a functional food that is rich in angiotensin I-converting enzyme inhibitors activity (ACE-inhibitor activity) and is antihypertensive. A completely randomized design was used in this study with six durations of fermentation (6, 12, 18, 24, 30, and 36 h) as treatments. Six hundred eggs obtained from the same chicken farm were used in the experiment as sources of egg albumen. Bacteria L. plantarum FNCC 0027 used in the fermentation was isolated from cowŌĆÖs milk. The parameters measured were the total bacteria, dissolved protein, pH, total acid and the activity of ACE-inhibitors. The results showed that there were significant effects of fermentation time on the parameters tested. Total bacteria increased significantly during fermentation for 6, 12, 18, and 24 h and then decreased with the increasing time of fermentation to 30 and 36 h. Soluble protein increased significantly during fermentation to 18 h and then subsequently decreased during of fermentation to 24, 30, and 36 h. The pH value decreased markedly during fermentation. The activities of ACE-inhibitor in fermented egg albumen increased during fermentation to 18 h and then decreased with the increasing of the duration of fermentation to 24, 30, and 36 h. The egg albumen which was fermented for 18 h resulted in a functional food that was rich in ACE-inhibitor activity.
Non-mammalian eggs, especially poultry eggs are an excellent natural food because the yolk and albumen contain complete nutrients required for the growth and development of a zygote (fertilized egg) to become an embryo and finally a healthy chick. The composition of yolk in the henŌĆÖs egg consists of approximately 33% lipid and 17% protein while the egg albumen is a source of water, proteins and minerals to embryo during development (Johnson, 2000; Kassis et al., 2010).
Eggs are not only used as a staple food, but also are used in food industries in the manufacture of cakes, breads, sweets and mayonnaise. Eggs are used as a complement in the food industry in the making of meatballs, sausage and ice cream. The diversity of the use egg albumen is associated with its functional properties that play a role in the processing food industry, such as a thickener and foaming agent because the egg albumen is easy to coagulate and form a foam (Watson, 2002; Murwani, 2012). Protein in the egg albumen contains amino acids and bioactive compounds that are beneficial to human health. One of these is angiotensin converting enzyme inhibitor (ACE-inhibitor) that has an antihypertensive effect by inhibiting the activity of angiotensin converting enzyme that increases blood pressure (Liu et al., 2010; Yu et al., 2011). However, the availability of ACE-inhibitor compounds is low in the egg albumen protein. Therefore, a process that increases the content of ACE-inhibitor in egg albumen is needed. An approach used is to parse the protein using an enzyme hydrolysis method and microbial fermentation.
Fermentation technology is used to obtain functional foods for health that improves nutrition, facilitates digestion and absorption and extends the shelf life of the products (Solomons, 2002; Li, 2010; Nahariah et al., 2013). Another benefit of fermentation is that it adds to the volume of production, is a low energy production process resulting in less amount of waste and does not pollute the surrounding environment (Haq et al., 2003; Nahariah et al., 2013).
Fermentation methods use proteolytic bacteria that can break down proteins. The bacteria used for fermentation of meat and milk is L. helveticus (Fuglsang et al., 2003; Isnafiah, 2003) and L. plantarum is used for the production of bekasem, a fermented fish product that rich in activity of ACE-inhibitors (Wikandari et al., 2011). Fermentation using L. helveticus in skim milk can produce ACE-inhibitor compounds (Sun et al., 2009).
The use of L. fermentum to ferment marine shrimp also produces ACE-inhibitor activity in the finished product (Wang et al., 2008). The use of bacteria such as Lactobacillus species; L. helveticus, L. bulgaricus, L. plantarum or a combination of these or some other Lactobacillus species has been carried out on food products but studies regarding fermentation using L. plantarum in egg albumen with a variety of fermentation times is limited. Therefore, the present study was conducted to evaluate the effect of fermentation time on egg albumen and the potential of fermented egg albumen as a functional food rich in ACE-inhibitor.
The materials used were 600 chicken eggs obtained from the same farm. There were 6 treatments of 20 chicken eggs each with 5 replicates. The lactic acid bacterial (LAB) culture was L. plantarum 0027 FNCC (Food and Nutrition Culture Collection) strains isolated from milk, Inter University Center Food and Nutrition, Gadjah Mada University, Yogyakarta, Indonesia). A completely randomized design was used with six durations of fermentation as a treatment i.e., 6, 12, 18, 24, 30, and 36 h.
The lactic acid bacteria (LAB) L. plantarum FNCC 0027 culture stock were stored on media de Man ROGOSA Sharpe order (MRS) agar. Preparation of sub-cultures were made by taking 1 dose (inoculating loop) of culture stock incorporated into MRS Broth liquid (Lactobacillus MRS Broth, M369-500 g, HIMEDIA, Mumbai, India) medium to which was added 20% tomato extract and further incubated for 24 h (Pramono et al., 2003). The sub-culture was inoculated into egg albumen with an added 20% tomato extract and further incubated for 24 h to obtain a working culture (Nahariah et al., 2013).
Egg albumen was separated from the yolks and stirred for 3 minutes without forming foam, ultraviolet sterilized by placing it on a portable clean room (PCR) hood (UV Sterilisation Cabinet) (GLE-UVSC-in version 09-05,22 Cambridge Science Park, Milton Road, Cambridge, UK) for 15 minutes. The working culture was added and homogenized with a tube shaker, then fermented at 37┬░C incubator (Ecocell LSIS-B2V/EC III SERIAL D 112456, MMM Medcenter Einrichtungen GmBH Semmelweisstrasse 6, Planegg, Germany) according to the duration of fermentation in the treatment.
Total lactic acid bacteria were calculated by the pour plate method (Irianto, 2010). A total of 1 mL of egg albumen sample diluted in 9 mL of sterile 0.86% BPW (Buffer Peptone Water, CM0509. OXOID, Ltd., Basingstoke Hampshire, England) and homogenized using a tube shaker. A series of dilutions from 10ŌłÆ1 to 10ŌłÆ7 were prepared using a sterile solution of 0.86% BPW. Each dilution was plated in a petri dish containing agar (Nutrient Agar, 500 g CM0003 OXOID, Ltd., Basingstoke Hampshire, England) and incubated at 37┬░C for 24 hours. Calculation of total bacteria was determined on the petri dish where the number of colonies ranged from 25 to 250. Total bacterial testing performed was 5 replicates/treatment.
The pH was measured by using a pH meter (Schott Instrument Lab 850, D-55122, Mainz, Germany). The egg albumen samples were measured without dilution (AOAC, 1984) by placing electrode in the sample. Before the pH was measured, the pH meter was calibrated with buffers used to buffer pH 4 and pH 7.
Measurement of total acid was conducted by titration method (AOAC, 1984). Ten milliliters of suspension plus three drops of phenolphthalein indicator were mixed then titrated with 0.1 N NaOH solution. The total acid titration was calculated by the formula: total acid (%) = (mL NaOH├Ś N NaOH├Ś1/10├Ś90)/mL sample.
A total of 0.1 mL of trichloroacetic acid-soluble extract sample was added 3.9 mL of distilled water, and as much as 5.5 mL Lowry reagent was added. The mixture was homogenized with a centrifuge (Type MX-305, Tomy Seiko, Co. Ltd., Tokyo, Japan) and incubated at room temperature for 10 minutes. Then 0.5 mL of Folin reagent was added and incubated at room temperature for 30 minutes, until the blue color was formed. Subsequently, the sample absorbance was measured at a wavelength of 600 nm. A solution of Bovine Serum Albumin (Product D0024 50 g, Bio Basic Canada Inc., Markhan Ontario, Canada) was used as a standard solution (Wikandari et al., 2011).
Analysis to evaluate the results of ACE-inhibitor activity in fermented egg albumen included: a solution of borate buffer (100 mM, pH 8.3) containing 300 mM NaCl added to the egg albumen fermentation. The same buffer used for the substrate was also used for the enzyme. Total reaction volume of 60 mL of 100 mM borate consisted of buffer (pH 8.3), 4 mML-histidyl-hippuryl-L-leucine (H1635-100 mg Sigma Aldrich Co 3050, product of USA), 300 mM NaCl, and 10 milli unit of ACE (A6778-1UN, Angiotensin converting enzyme from rabbit lung, Sigma Aldrich, St. Louise MO, USA). The individual chemicals were incubated at 37┬░C for 30 minutes in a waterbath before mixing, and an additional 30 minutes at the same temperature after mixing the ingredients. The reaction was stopped by adding 60 mL of 1 M HCl and was then tested by using a spectrophotometer (Perkin Elmer UV Winlab US-710 CT 06484-4794, Shelton, CT, USA) to measure hippuric acid produced by enzymatic hydrolysis of the substrate hippuryl - L- histidyl - L ŌĆō leucine at a wavelength of 228 nm (Liu et al., 2010). The ACE-inhibitor activity (%) = ([AŌĆōB]/AŌĆōC)├Ś100, where A is the control (reaction buffer solution, enzyme ACE without sample and added HCl before incubation 37┬░C), B is the sample (reaction buffer, enzyme ACE with egg albumen fermentation) and C is a blank (reaction buffer solution, enzyme ACE without sample and added HCl after incubation at 37┬░C).
Data were analyzed for variance (Steel and Torrie, 1991) based on completely randomized design. The test was used to determine the pattern of changes in the value of each variable of the fermentation time by Duncan test to determine treatment differences. Data were analyzed by using SPSS software.
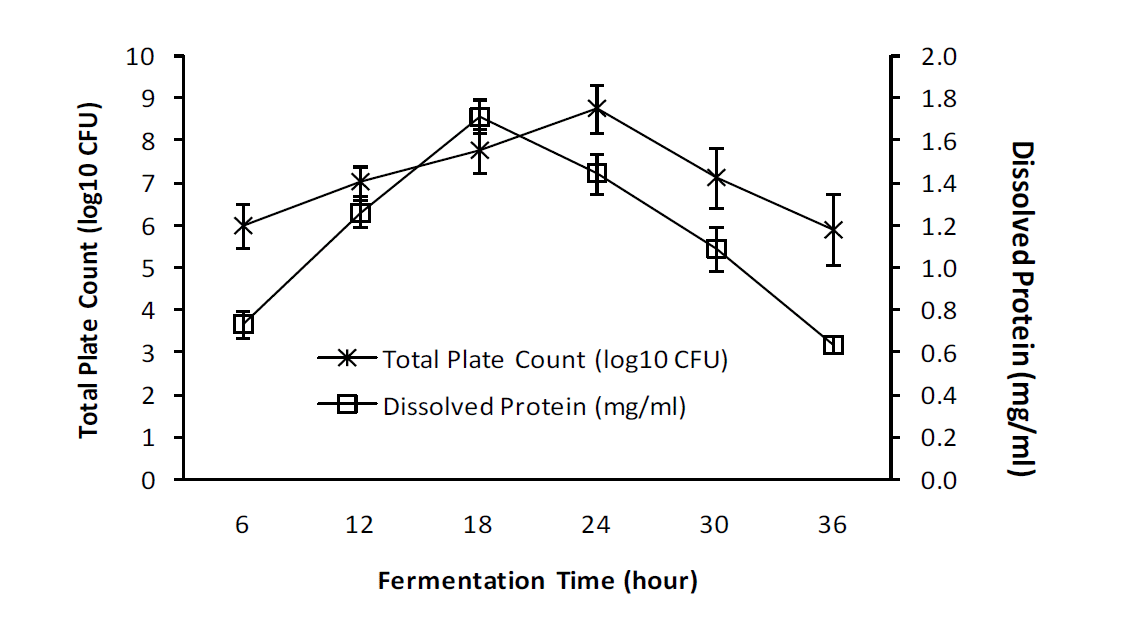
The results of statistical analysis showed that the duration of fermentation significantly affected (p = 0.000) the total microbial content of fermented egg albumen (Figure 1). Total microbial (log 10 colony-forming unit [CFU]) content of the fermented egg albumen increased with increasing duration of fermentation and reached the peak level during 24 hours (8.76┬▒0.555) of fermentation. Total microbial content of the fermented egg albumen decreased with an increasing fermentation time from 24 hours to 30 hours and reached the lowest level following 36 hours (5.90┬▒0.842) of fermentation. There was no real difference in total microbial contents among duration of fermentation of 12 h (7.02┬▒0.386), 18 hours (7.76┬▒0.532) and 30 h (7.15┬▒0.717). The total microbial contents of fermented egg albumen during 36 h decreased to the low level similar to those found during 6 h (5.99┬▒0.509) fermentation.
Protein dissolved in the egg albumen showed a very significant increase (p = 0.000) with the increase in fermentation time (Figure 1) from 6 (0.73┬▒0.067 mg/mL) to 12 hours (1.27┬▒0.18 mg/mL) and reached the highest level after 18 h (1.72┬▒0.148 mg/mL) of fermentation. Protein dissolved in fermented egg albumen started to decrease after 24 h (1.44┬▒0.097 mg/mL) to reach its lowest level after 36 h (0.63┬▒0.039 mg/mL) of fermentation. There was a significant difference in protein dissolved in fermented egg albumen between fermentation times of 6, 12, and 18 h. However, there was no difference in protein dissolved in fermented egg albumen after 12, 24, 30, and 36 h of fermentation.
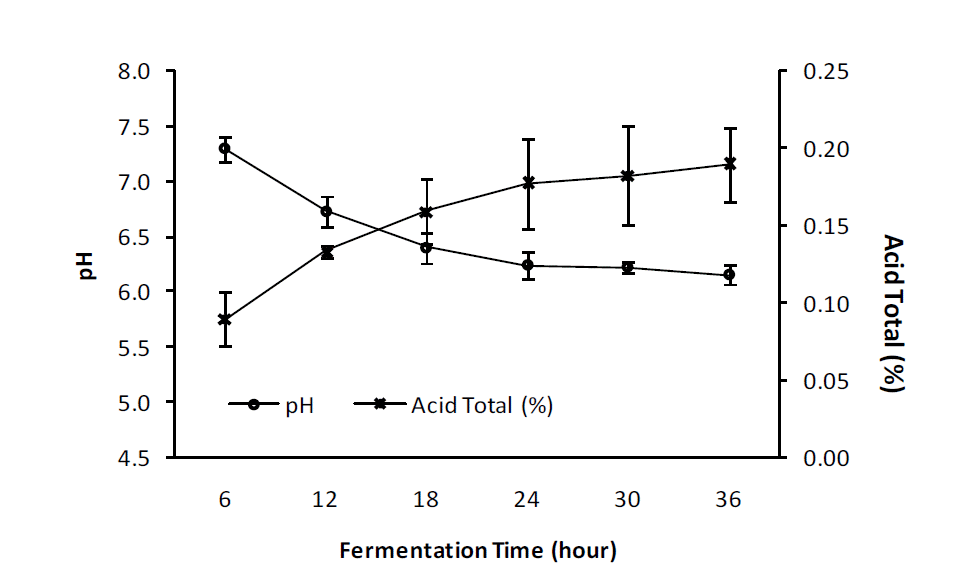
The results of the study (Figure 2) showed that the increasing of duration of fermentation using L. plantarum significantly (p = 0.000) increased the total acids in fermented egg albumen. There is real difference in the percentage of total acids in fermented egg albumen during 6 h (0.09┬▒0.017) to 12 h (0.13┬▒0.004) of fermentation, but both were significantly different from those following 18 h (0.16┬▒0.021), 24 h (0.18┬▒0.029), 30 h (0.18┬▒0.032) and 36 h (0.19┬▒0.024) fermentation. The increasing of duration of fermentation to 24, 30, and 36 h did not significantly increase total acid compared to 18 h of fermentation.
The results of the study (Figure 2) showed that, the increasing of duration of fermentation with L. plantarum significantly (p = 0.000) decreased the pH of fermented egg albumen. The pH values of fermented egg albumen decreased markedly with fermentation times from 6 h (7.30┬▒0.077), 12 (0.13┬▒0.004) to 18 h (6.40┬▒0.082). However, further fermentation to 24 h (6.24┬▒0.125), 30 h (6.22┬▒0.050) and 36 h (6.15┬▒0.081) did not affect the pH of the fermented egg albumen.
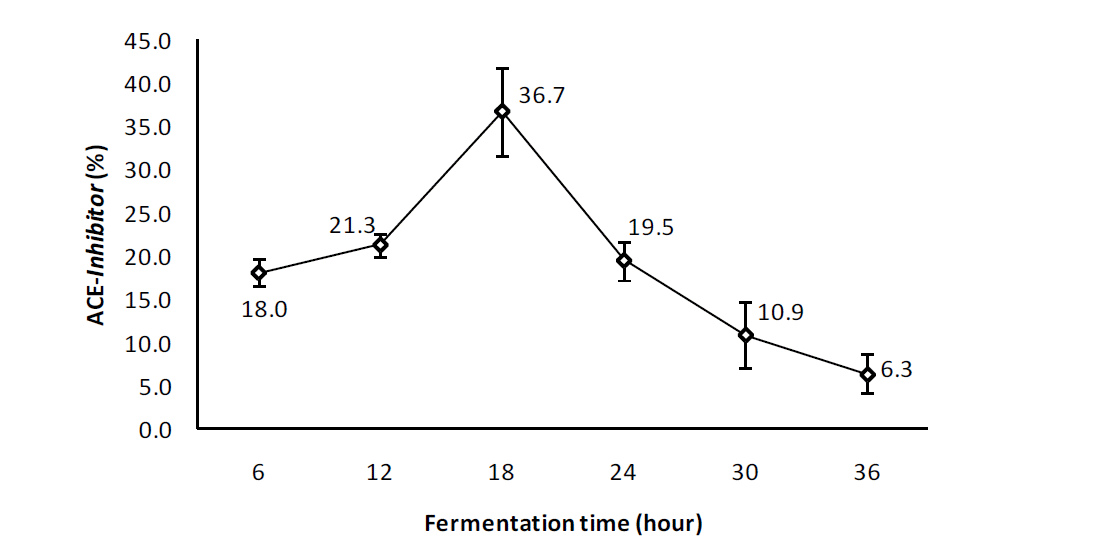
The potential of egg albumen as a food that is rich in ACE-inhibitor activity can be seen in Figure 3. The results showed that the increasing of duration of fermentation significantly (p = 0.000) from 6 h (18.0┬▒1.24) to 12 h (21.3┬▒1.75) increased the ACE-inhibitor activity and reached the highest level after 18 h (36.7┬▒3.33) of fermentation by L. plantarum. Increasing the time of fermentation to 24 h (19.5┬▒2.57) and 30 h (10.9┬▒2.75) decreased ACE-inhibitor activity in the egg albumen until it reached the lowest level after 36 h (6.30┬▒1.66) of fermentation. The pattern of ACE-inhibitor activity was similar to that of dissolved protein in the fermented egg albumen.
Egg albumen fermented for 6 h (5.99┬▒0.509) to 24 h (8.76┬▒0.555) had an increase the population of L. plantarum since the egg albumen rich in nutrients. Egg albumen contains essential amino and non-essential acids (Kassis et al., 2010), carbohydrates, and fat, although in small quantities (Bell and Weaver, 2002), and these compounds provide component N, S, O, and C, which are necessary for the growth of microbia (Molin, 2008; Nisa et al., 2008). It provides an appropriate environment to improve the productivity of bacterial fermentation of biomass described as output per unit of time (Stanbury et al., 2003; Irianto, 2010). Fermentation for 30 h (7.15┬▒0.717) and 36 h (5.90┬▒0.842) showed a significant decline in the number of bacteria due to the possibility that the availability of nutrients in the egg albumen began to decrease, so that the bacteria died as was characterized by the decrease of colonies formed during fermentation. The duration of fermentation affects the number of microbes that can grow (Sun et al., 2009; Nahariah et al., 2013). Egg albumen fermented using L. plantarum for 24 h resulted in a total of 109 microbes and this value was higher than that found in fermented fish bekasem i.e., 105 to 108 CFU/mL after 6 days of fermentation (Wikandari et al., 2011). Similarly it is higher than the research of Nahariah et al. (2013), who reported a total of 106 CFU/mL microbes in egg albumen as a growing medium for L. plantarum during 24 h of fermentation.
In general, fermentation breaks down carbohydrates in food while food containing protein requires a certain type of bacteria, such as L. plantarum, that can degrade protein (Kurtis, 2008) by proteolytic activity to produce amino acids (Setioningsih et al., 2004; Nisa et al., 2008). A similar study using L. plantarum bacteria in bekasem fish reported an increase in soluble protein of 0.46 mg/mL to 0.81 mg/mL after 9 days of fermentation (Wikandari et al., 2011). Figure 1 shows the pattern of the increasing number of L. plantarum during 6 to 18 h of fermentation, followed by the increased concentration of dissolved protein. Through 24 h of fermentation, the total number of bacteria increases, but the amount of the dissolved protein decreased. The optimum fermentation time to obtain dissolved proteins is 18 h. This suggests that the ability of L. plantarum to digest the egg albumen protein at 18 h of fermentation is associated with the exponential growth of L. plantarum during this period.
An increase in total acid in fermented egg albumen during the fermentation process is associated with the possibility of an increase in the number of bacteria that can break down carbohydrate and protein compounds into simpler compounds, including acid and water (Desrosier, 1988). This study showed a slightly higher total acid (0.09% to 0.19%) as compared to that reported in egg albumen fermented for the purpose of obtaining an inoculum (0.08 to 0.17) (Nahariah et al., 2013), and that produced in fish bekasem (0.18%) (Wikandari et al., 2011). Figure 2 shows the decreasing pH with the increasing of total acid in the fermented egg albumen with the increasing time of fermentation. This suggests that the bacterial activity in the egg albumen produced the acidic conditions that lower the pH as L. plantarum is able to break down complex compounds into simpler compounds that produce lactic acid in the substrate (Francois et al., 2007; Nursyam, 2011; Sutrisna, 2013).
ACE-inhibitor is a protein or peptide that exists in food but in general is not in an active state and therefore requires enzymatic or fermentation processes to break down the proteins (Vinderola et al., 2008; Akillioglu and Karakaya, 2009). ACE-inhibitor activity increases due to an increasing concentration of proteins and soluble peptides by L. plantarum during the fermentation process. However, the results obtained in this study were lower than the 51.77% increase reported by Wikandari et al. (2011) in fish bekasem fermented for 6 days. The use of bacteria L. fermentum in marine shrimp fermented for 24 h at a temperature of 38┬░C resulted in ACE-inhibitor activity (IC50) of 3.37 mg/mL (Wang et al., 2008), and enzyme hydrolysis using alcalase on egg albumen for 180 minutes produced an increase of ACE-inhibitor by 58% (Liu et al., 2010). The ACE-inhibitor activity in chicken leg bone degraded using alcalase enzyme hydrolysis increased by 84.33% after 4 h hydrolysis, and reached a 75.53% increase when it was hydrolysed by pepsin for 6 h and when hydrolysed with trypsin for 8 h the increase 77.86% (Cheng et al., 2008). However, extraction of the legume protein by the method of heating produced ACE-inhibitor activity increase of 4.08% to 28.54% (Akillioglu and Karakaya, 2009), which was lower than bacterial fermentation with L. plantarum of egg albumen.
The utilization of L. plantarum to hydrolyze the protein in egg albumen showed lower ACE-inhibitor activity compared to that reported in other studies. Egg whites fermented for 18 h using L. plantarum produced an ACE-inhibitor activity increase of 36.7┬▒3.33%, lower than an increase of 51.77% ACE-Inhibitor activity following a 6 day fermentation using L. plantarum bacteria in fish bekasem (Wikandari et al., 2011). Liu et al. (2010) reported an increase in ACE-inhibitor activity of 58% using enzyme alcalase. The present lower result was likely due to the ability of the bacterium L. plantarum to break down more complex compounds that exist in egg albumen requiring a longer adaptation time (Nahariah et al., 2013). In addition, egg albumen has a 0.3% glucose availability (Bell and Weaver, 2002) so there were less nutrient sources available for L. plantarum. Egg albumen is mostly composed of simple proteins and protein complexes such as ovalbumin, ovoglobulin, ovoconalbumin and glycoproteins including ovomucin and ovomucoid (Romanoff and Romanoff, 1963) and the presence of anti-bacterial lysozyme as likely to be a factor limiting the growth of bacteria L. plantarum. Another possibility is that L. plantarum on egg albumen breaks down protein into amino acids and peptides for use as a sources of nitrogen for the growth and multiplication of cells (Nisa et al., 2008) thus reducing peptides ACE-inhibitor peptides.
There is an increase in the total number of bacteria L. plantarum, soluble protein, total acid, ACE-inhibitor activity and a decrease in pH value during fermentation of egg albumen. Egg albumen fermented for 18 h had a 40% increase in ACE-inhibitor activity (antihypertensive) and showed potential as a healthy food ingredient.
ACKNOWLEDGMENTS
The authors are grateful for the assistance of Yuny Erwanto, Prima Wikandari, Hikmah Muhammad Ali Wasmen Manalu, and Edy Kurnianto in processing of data and reading of the manuscript.
REFERENCES
Akillioglu HG, Karakaya S. 2009. Effects of heat treatment and in vitro digestion on the angiotensin converting enzyme inhibitory activity of some legume species. Eur Food Res Technol 229:915ŌĆō921.

AOAC. 1984. Official Methods of Analysis. 12th edAssociation of Official Analysis Chemist; Washington, DC, USA:
Bell DD, Weaver WD. 2002. Commercial Chicken Meat and Egg Production. Kluwer Academic Publishers; Norwell, MA, USA:
Cheng FY, Liu YT, Wan TC, Lin LC, Sakata R. 2008. The development of angiotensin I-converting enzyme inhibitor derived from chicken bone protein. Anim Sci J 79:122ŌĆō128.

Desrosier NW. 1988. Food Preservation Technology. Universitas Indonesia; Jakarta, Indonesia: (In Indonesian)
Francois ZN, Hodo NE, Florence FA, Paul MF, Felicite TM, Soda ME. 2007. Biochemical properties of some thermophilic lactic acid bacteria strain from traditional fermented milk relevant to their technological performance as starter culture. Biotechnology 6:14ŌĆō21.

Fuglsang A, Rattray FP, Nilsson D, Nyborg NCB. 2003. Lactic acid bacteria: Inhibition of angiotensin converting enzyme in vitro and in vivo. Antonie van Leeuwenhoek 83:27ŌĆō34.


Haq KH, Mukhtar H, Daudi S, Ali S, Qadeer MA. 2003. Production of protease by a locally isolated mould culture under lab condition. Biotechnology 2:30ŌĆō36.

Irianto K. 2010. Microbiology: Reveals the World of Microorganisms. Yrama Widya, Bandung; West Java, Indonesia: (In Indonesian)
Isnafia JA. 2003. Characteristics of beef fermented by Lactobacillus plantarum and naturally fermented. Med Pet 26:40ŌĆō43.
Johnson AL. 2000. Reproduction in the Female. SturkieŌĆÖs Avian Physiology. Whittow GC, editorAcademic Press; San Diego, California, USA:

Kassis NM, Beamer SK, Matak KE, Tou JC, Jaczynsky J. 2010. Nutritional composition of novel nutraceutical egg products developed with omega-3-rich oil. LWT-Food Technol 43:1204ŌĆō1212.

Katayama K, Fuchu H, Sakata A, Kawahara S, Yamauchi K, Kawamura Y, Muguruma M. 2003. Angiotensin I-converting enzyme inhibitory activities of porcine skeletal muscle proteins following enzyme digestion. Asian Australas J Anim Sci 16:417ŌĆō424.

Kurtis AE. 2008. Fermentation. 1: Biotech processes. J Validation Technol 14:10ŌĆō16.
Li S. 2010. Reduce the IgE Binding Ability of Egg White Protein by Fermentation. PhD Thesis. University of Alberta; Edmonton, Alberta, Canada:
Liu JB, Yu ZP, Zhao WZ, Lin SY, Wang EL, Zhang Y, Hao H, Wang ZZ, Chen F. 2010. Isolation and identifikasi of angiotension-converting enzyme inhibiting peptide from egg white protein hydrolysates. Food Chem 122:1159ŌĆō1163.

Murwani R. 2012. Functional ingredients from egg. Functional Foods. Food Review Indonesia 7:28ŌĆō30.
Molin G. 2008. Lactobacillus plantarum The Role in Foods and in Human Health. Handbook of Fermented Functional Foods. Farnworth ER, editorCRC Press; Boca Raton, FL, USA: 978-1-4200-5326-5
Nahariah N, Legowo AM, Abustam E, Hintono A, Pramono dan YB, Yuliati FN. 2013. The growth of Lactobacillus plantarum on eggs white with different fermentation time. J Anim Sci Technol 3:33ŌĆō39. (In Indonesian)
Nisa FC, Kusnadi J, Chrisnasari dan R. 2008. Viability and sublethal detection of probiotic bacteria in fermented soy milk instant using freeze-drying method (Study type isolates and the concentration of sucrose as cryoprotectant). J Agric Technol 9:40ŌĆō51. (In Indonesian)
Nursyam H. 2011. PH value, total acid, N-total and N-Amino of tuna (Thunnus sp) sausage was fermented by Lactobacillus plantarum. Sci J Fish Mar 3:221ŌĆō228. (In Indonesian)
Pramono YB, Harmayani E, Utami T. 2003. Growth kinetics of Lactobacillus plantarum and Lactobacillus sp in liquid MRS medium. Technol Food Ind J 14:46ŌĆō50.
Romanoff AL, Romanoff AJ. 1963. The Avian Egg. John Wileydan Sons Inc.; New York, NY, USA:
Setioningsih E, Setyaningsih R, Susilawati dan A. 2004. Making of probiotic beverage from soybean milk using inoculum of Lactobacillus casei, Lactobacillus plantarum, and Lactobacillus acidophilus. Biotechnol 1:1ŌĆō6. (In Indonesian)
Solomons NW. 2002. Fermentation, fermented foods and lactose intolerance. Eur J Clin Nutr 56:S50ŌĆōS55.

Stanbury PF, Whitaker A, Hall SJ. 2003. Principles of Fermentation Technology. Butterworth Heinemann; Oxford, UK:
Steel RGD, Torrie JH. 1991. Principle and Procedure of Statistics. 2nd edInternational Book Company; Tokyo, Japan:
Sun ZH, Liu WJ, Zhang JC, Yu J, Gao W, Jiri M, Menghe B, Sun TS, Zhang HP. 2009. Identification and characterization of the dominant lactic acid bacteria isolated from traditional fermented milk in mongolia. Folia Microbiol 55:270ŌĆō276.

Sutrisna R. 2013. Characteristics of lactic acid bacteria isolated from duck intestines against Escherichia coli and Salmonella pullorum. In : Conference on Science and Technology V; Research Institute of the University of Lampung, Indonesia. November 19ŌĆō20 2013; (In Indonesia)
Vinderola G, LeBlanc AM, Perdigon G, Matar C. 2008. Biologically Active Peptides Released in Fermented Milk. Handbook of Fermented Functional Foods. Farnworth ER, editorCRC Press; New York, NY, USA:
Wang YK, He HL, Chen XL, Sun CY, Zhang YZ, Zhou BC. 2008. Production of novel angiotensin I-converting enzyme inhibitory peptides by fermentation of marine shrimp Acetes chinensis with Lactobacillus fermentum SM 605. Appl Microbiol Biotechnol 79:785ŌĆō791.


Watson RR. 2002. Egg and Health Promotion. Iowa State Press A: Blackwell Publishing Company; 0-8138-2798-1
Wikandari PR, Suparmo , Marsono Y, Rahayu ES. 2011. Bekasem as a potential source of angiotensin I-converting enzyme inhibitor. Biota 16:142ŌĆō152.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





