Antunes RC. 2007. Planejando a reposição de reprodutores (macho e fêmea) e impacto sobre a eficiência reprodutiva da granja. Rev Bras Reprod Anim 31:41–46.
Baxter EM, Jarvis S, D’Eath RB, Ross DW, Robson SK, Farish M, Nevison IM, Lawrence AB, Edwards SA. 2008. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 69:773–783.


Brown-Brandl TM, Eigengerg RA, Nienaber JA, Kachman SD. 2001. Thermoregulatory profile of a newer genetic line of pigs. Livest Prod Sci 71:253–260.

Damgaard LH, Rydhmer L, Lovendahl P, Grandinson K. 2003. Genetic parameters for within-litter variation in piglet birth weight and change in within-litter variation during suckling. J Anim Sci 81:604–610.


Ferreira RA, Chiquieri J, Mendonça PP, Melo TV, Cordeiro MD, Soares RTRN. 2007. Comportamento e parâmetros fisiológicos de leitões nas primeiras 24 horas de vida. Ciênc Agrotec 31:1845–1849.

Gondret F, Lefaucheur L, Louveau I, Lebret B, Pichodo X, Le Cozler Y. 2005. Influence of piglet birth weight on postnatal growth performance, tissue lipogenic capacity and muscle histological traits at market weight. Livest. Prod Sci 93:137–146.

Herpin P, Le Dividich J. 1995. Thermoregulation and the environment. The Neonatal Pig. Varley M, editorDevelopment and SurvivalCAB International; Wallingford: p. 57–98.
Herpin P, Damon M, Le Dividich J. 2002. Development of thermoregulation and neonatal survival in pigs. Livest Prod Sci 78:25–45.

Herpin P, Vincent A, Damon M. 2004. Effect of breed and body weight on thermoregulatory abilities of European (Pietrain×(Landrace×Large White)) and Chinese (Meishan) piglets at birth. Livest Prod Sci 88:17–26.

Jensen T, Pedersen LJ, Jorgensen E. 2011. Hypothermia in neonatal piglets: Interactions and causes of individual differences. J Anim Sci 89:2073–2085.


Júnior DC, Matteri RL, Carroll JA, Fangman TJ, Safranski TJ. 2002. Preweaning survival in swine. J Anim Sci 80:E74–E86.

Lima AL, Oliveira RFM, Donzele JL, Fernandes HC, Campos PHRF, Antunes MVL. 2011. Resfriamento do piso da maternidade para porcas em lactação no verão. Rev Bras Zootec 40:804–811.

Lossec G, Herpin P, Le Dividich J. 1998. Thermoregulatory responses of the newborn pig during experimentally induced hypothermia and rewarming. Exp Physiol 83:667–678.


Malmkvist J, Pedersen LJ, Damgaard BM, Thodberg K, Jorgensen E, Labouriau R. 2006. Does floor heating around parturition affect the vitality of piglets born to loose housed sows? Appl Anim Behav Sci 99:88–105.

Manno MC, Oliveira RFM, Donzele JL, Ferreira AS, Oliveira WP, Lima KRS, Vaz RGM. 2005. Efeito da temperatura ambiente sobre o desempenho de suínos dos 15 aos 30 kg. R Bras Zootec 34:1963–1970.

Mendonça AB. 2010. Conforto térmico em suínos visando melhoria na produção e qualidade do produto final. Postgraduate Monography. Universidade Castelo Branco; Campinas, SP, Brazil:
Merks J, Ducro-Steverink D, Feitsma H. 2000. Management and genetic factors affecting fertility in sows. Reprod Domest Anim 35:261–266.

Orozco-Gregorio H, Mota-Rojas D, Alonso-Spilsbury M, Gonzalez-Lozano M, Trujillo-Ortega M, Olmos-Hernandez SA, Sanchez-Aparicio P, Ramãrez-Necoechea R, Hernandez-Gonzalez R, Uribe-Escamilla R, Villanueva-Garcia D. 2007. Importance of blood gas measurements in perinatal asphyxia and alternatives to restore the acid base balance status to improve the newborn performance. Am J Biochem Biotechnol 3:131–140.

Pandorfi H, Silva IJO, Moura DJ, Sevegnani KB. 2005. Microclima de abrigos escamoteadores para leitões submetidos a diferentes sistemas de aquecimento no período de inverno. Rev Bras Eng Agric Amb 9:99–106.

Panzardi A, Bierhals T, Mellagi APG, Bernardi ML, Bortolozzo FP, Wentz I. 2009. Survival of piglets according to physiological parameters at birth. In : Proceedings of the 8th International Conference on Pig Reproduction; Banff, Canada. (in press)
Panzardi A, Bernardi ML, Mellagi AP, Bierhals T, Bortolozzo FP, Wentz I. 2013. Newborn piglet traits associated with survival and growth performance until weaning. Prev Vet Med 110:206–213.


Pastorelli GM, Neil M, Wigren I. 2009. Body composition and muscle glycogen contents of piglets of sows fed diets differing in fatty acids profile and contents. Livest Sci 123:329–334.

Quesnel H, Farmer C, Devillers N. 2012. Colostrum intake: Influence on piglet performance and factors of variation. Livest Sci 146:105–114.

Quiniou N, Dagorn J, Gaudré DD. 2002. Variation of piglet’s birth weight and consequences on subsequent performance. Livest Prod Sci 78:63–70.

SAS Institute Inc. 2001. SAS/STAT user’s guide: Version 6. 6th edSAS Institute Inc; Cary, North Carolina:
Tuchscherer M, Puppe B, Tuchscherer A, Tiemann U. 2000. Earley identification of neonates at risktraits of newborn piglets with respect to survival. Theriogenology 54:371–388.


Van Rens BTTM, De Koning G, Bergsma R, Der Van Lende T. 2005. Preweaning piglet mortality in relation to placental efficiency. J Anim Sci 83:144–151.


Yan PS, Yamamoto S. 2000. Relationship between thermoregulatory responses and heat loss in piglets. J Anim Sci 71:505–509.


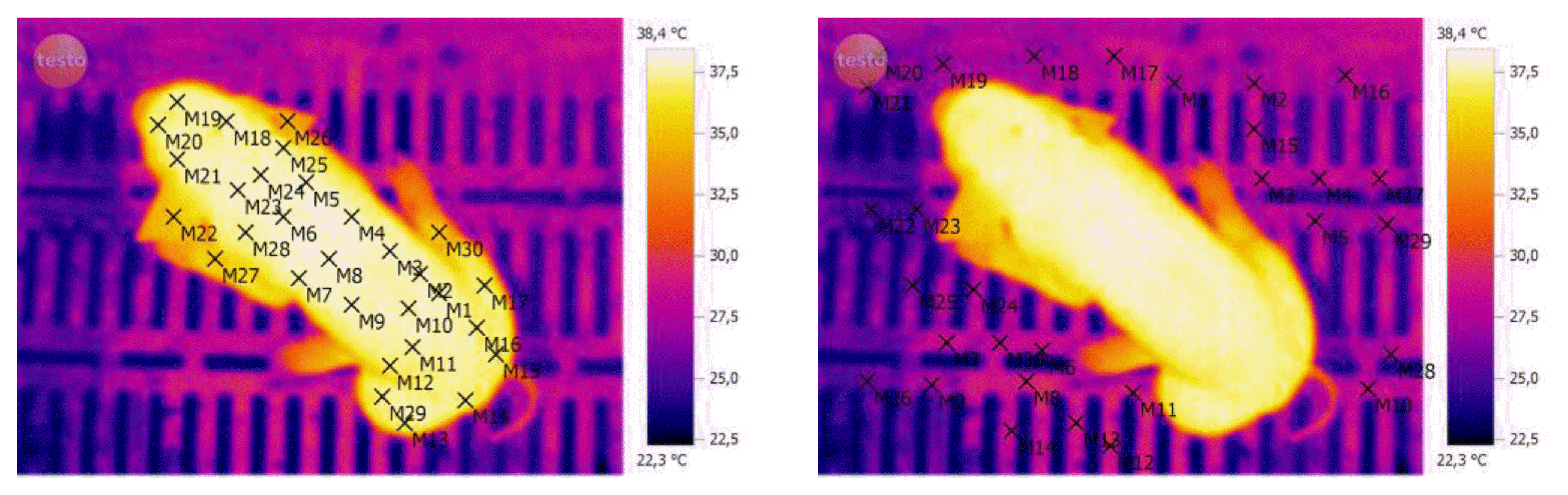
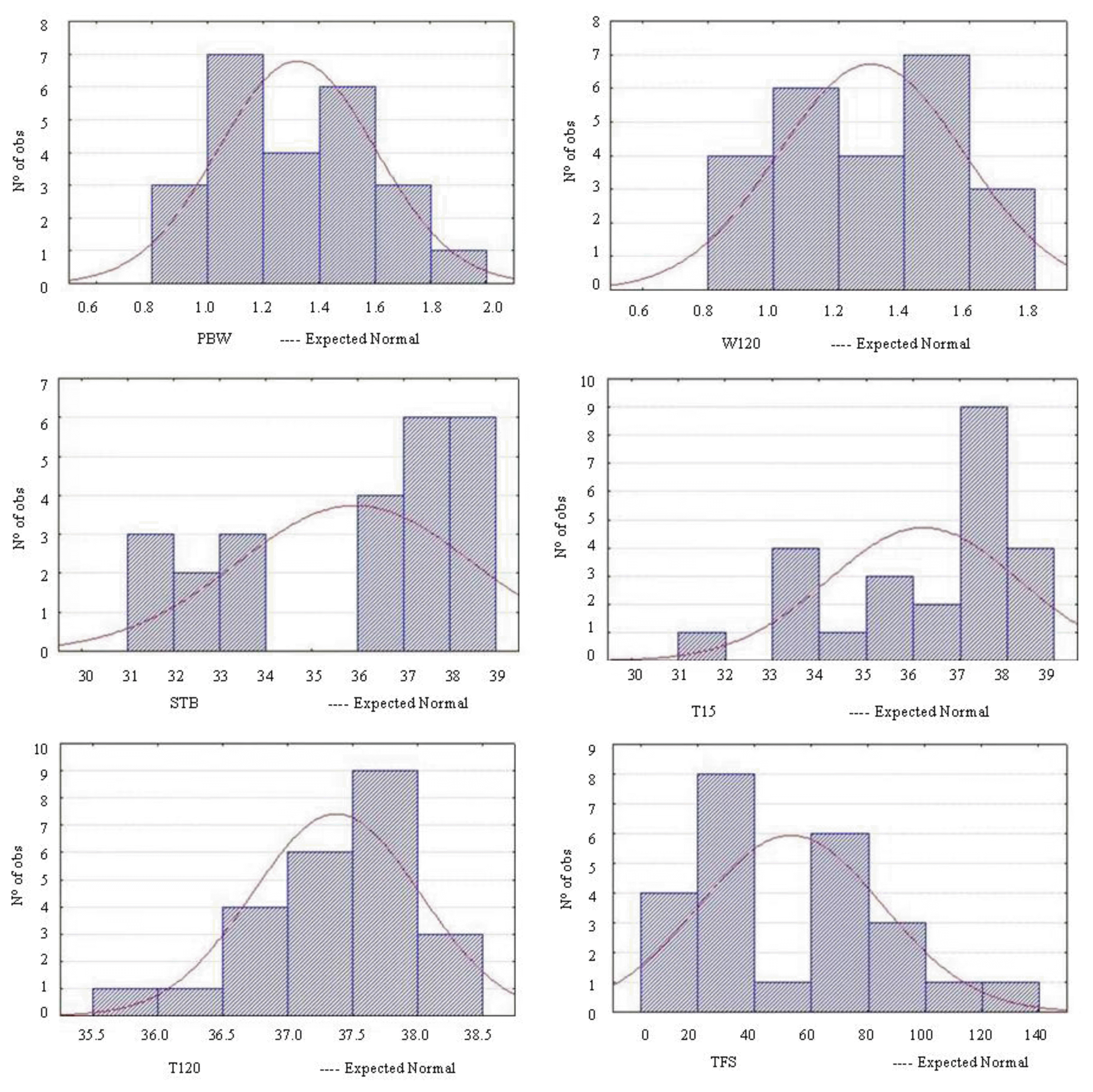
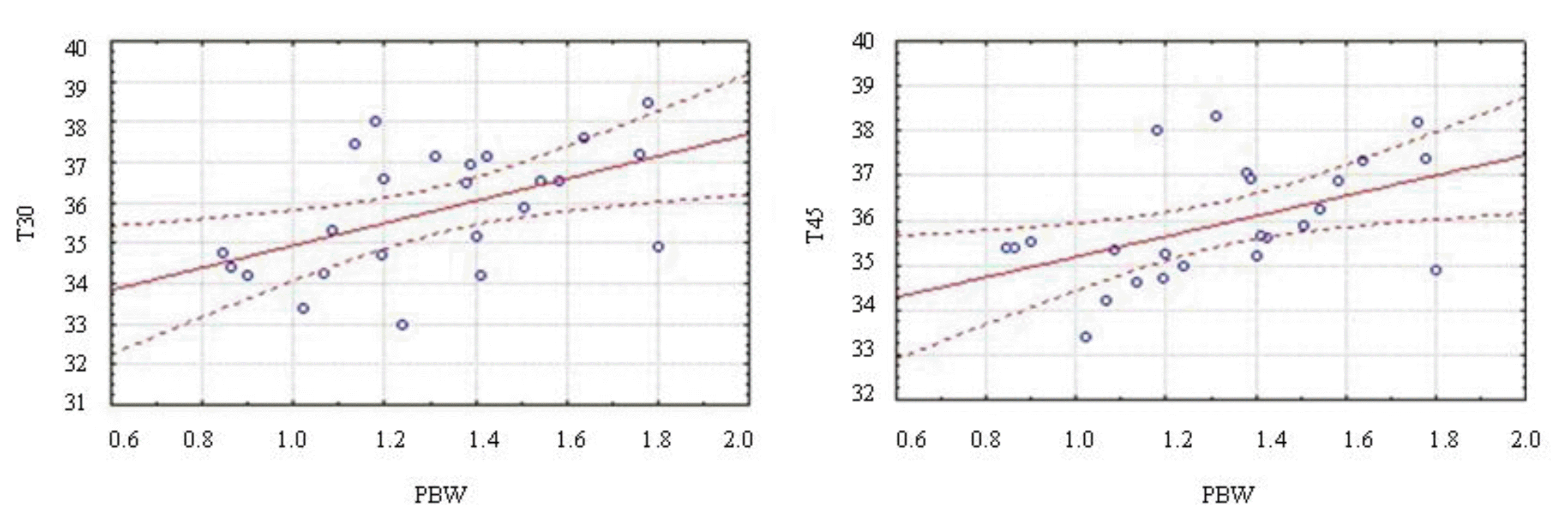
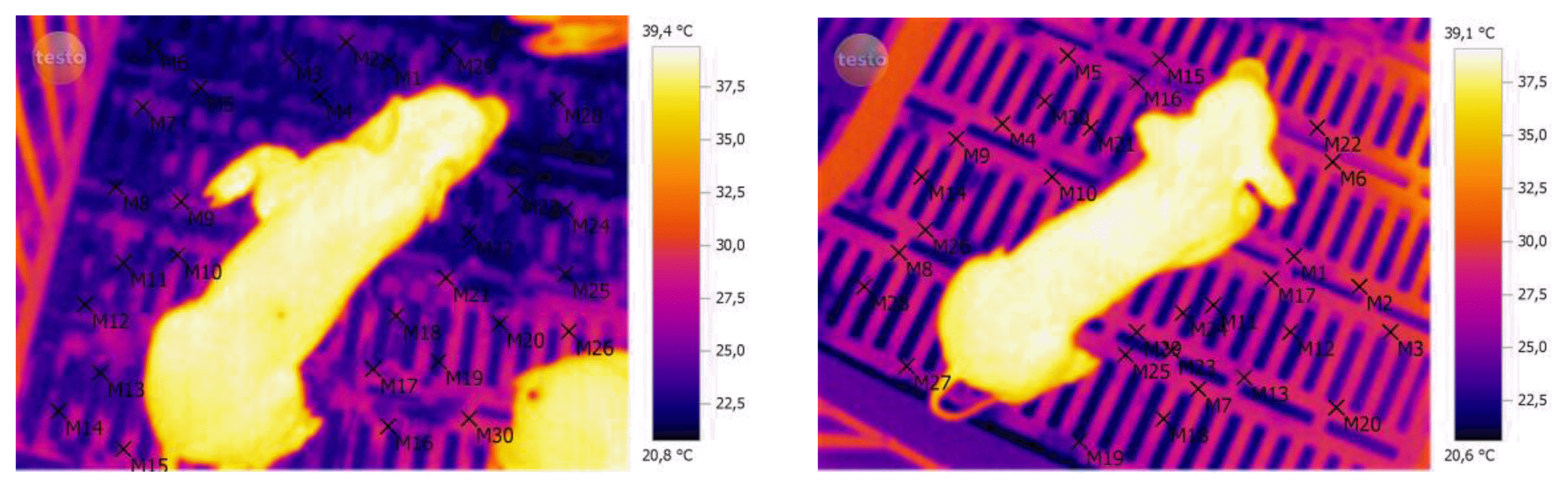





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





