 |
 |
| Anim Biosci > Volume 36(10); 2023 > Article |
|
Abstract
Objective
The objective of this study was to investigate the phylogenetic and expression analysis of the angiopoietin-like (ANGPTL) gene family and their role in lipid metabolism in pigs.
Methods
In this study, the amino acid sequence analysis, phylogenetic analysis, and chromosome adjacent gene analysis were performed to identify the ANGPTL gene family in pigs. According to the body weight data from 60 Jinhua pigs, different tissues of 6 pigs with average body weight were used to determine the expression profile of ANGPTL1–8. The ileum, subcutaneous fat, and liver of 8 pigs with distinct fatness were selected to analyze the gene expression of ANGPTL3, ANGPTL4, and ANGPTL8.
Results
The sequence length of ANGPTLs in pigs was between 1,186 and 1,991 bp, and the pig ANGPTL family members shared common features with human homologous genes, including the high similarity of the amino acid sequence and chromosome flanking genes. Amino acid sequence analysis showed that ANGPTL1–7 had a highly conserved domain except for ANGPTL8. Phylogenetic analysis showed that each ANGPTL homologous gene shared a common origin. Quantitative reverse-transcription polymerase chain reaction analysis showed that ANGPTL family members had different expression patterns in different tissues. ANGPTL3 and ANGPTL8 were mainly expressed in the liver, while ANGPTL4 was expressed in many other tissues, such as the intestine and subcutaneous fat. The expression levels of ANGPTL3 in the liver and ANGPTL4 in the liver, intestine and subcutaneous fat of Jinhua pigs with low propensity for adipogenesis were significantly higher than those of high propensity for adipogenesis.
Conclusion
These results increase our knowledge about the biological role of the ANGPTL family in this important economic species, it will also help to better understand the role of ANGPTL3, ANGPTL4, and ANGPTL8 in lipid metabolism of pigs, and provide innovative ideas for developing strategies to improve meat quality of pigs.
The angiopoietin-like protein (ANGPTL) family is a type of secretory protein that plays a variety of roles in lipid metabolism, glucose metabolism, energy metabolism, angiogenesis, and stem cell biology [1]. Excluding ANGPTL8, ANGPTL families are similar to Angiopoietins (ANGs) and share common characteristics, including the N-terminal signal sequence and coiled-coil domain (CCD), that mediates the formation of homo-oligomers, and the C-terminal fibrinogen domain (FReD) that regulates ligand activity [2]. ANGPTL8, an atypical member of the ANGPTL family, consists of 198 amino acids and lacks FReD in humans [3]. Disordered lipid metabolism will lead to cardiovascular disease, diabetes, and obesity, and it also affects the meat quality of animals [4].
The investigation on improving meat production as well as meat quality has been pursued by many animal husbandry researchers. Extensive studies confirm that ANGPTL3, ANGPTL4 and ANGPTL8 play an important role in lipid metabolism and are all lipoprotein lipase (LPL) inhibitors [5]. LPL was originally identified as a triglyceride (TG) scavenging factor lipase, it is produced by cardiomyocytes and adipocytes and is transported by GPIHBP1 protein to the lumen side of capillary endothelial cells [6], where it hydrolyzes TG into fatty acids for being absorbed into tissues.
ANGPTL3 is a liver-specific secretion factor and is highly expressed in the liver [7]. Its expression is regulated by the liver X receptor (LXR). The main effect of ANGPTL3 is to inhibit the activity of LPL in the capillaries of adipose tissue and muscle [8]. Intriguingly, ANGPTL3 plays a major role in promoting the uptake of very low density lipoprotein (VLDL)-derived TG into white adipose tissue rather than oxidative tissues such as skeletal muscle, heart brown adipose tissue in the feeding state [9].
ANGPTL4 is also known as fasting-induced adipokine (FIAF) or peroxisome proliferator-activated receptor gamma angiopoietin-related protein (PGAR) [10]. ANGPTL4 regulates lipid homeostasis and participates in regulating the intestinal microbiota in fat deposition [11]. Unlike ANGPTL3, ANGPTL4 can be secreted by adipose tissue, intestinal tissues, liver, skeletal muscle, heart, and other tissues [12]. ANGPTL4 is released as a 50 kD hormone precursor, which is then cut into N-terminal and C-terminal fragments, the N-terminus of ANGPTL4 acts as a lipoprotein lipase (LPL) inhibitor [12]. Research shows that ANGPTL4 is negatively correlated with low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol and is positively correlated with various lipid metabolic syndromes [13]. ANGPTL4 is also an endogenous inhibitor to intestinal fatty digestive enzymes. The knockout of ANGPTL4 in mice would increase the body weight, decrease the lipid content in feces, and increase dietary triglyceride accumulation in the small intestine, which was consistent with the increased intestinal lumen lipase activity [14]. ANGPTL3 mainly inhibits LPL activity in the feeding state, whereas ANGPTL4 plays an important role in both feeding and fasting states [15]. Different from ANGPTL3, ANGPTL4 is considered to be an endocrine or autocrine/paracrine inhibitor of LPL due to its different expression sites.
ANGPTL8 is a new protein mainly expressed in the human liver and plays an important role in regulation of plasma TG levels and lipid metabolism [16]. In addition, the N-terminal domain of ANGPTL8 is 20% identical to ANGPTL3 and ANGPTL4 and functionally binds to LPL to regulate triglyceride metabolism, indicating the affinity between them [17]. ANGPTL8 could interact with ANGPTL3, and they could synergistically inhibit LPL activity and regulate the plasma triglyceride levels [18]. Expression of ANGPTL8 in ANGPTL3 knockout mice failed to increase the levels of triacylglycerol non-esterified fatty acids in plasma [19]. Since ANGPTL3, ANGPTL4, and ANGPTL8 are all LPL inhibitors, the absence of anyone of them would break the balance of triglyceride metabolism in turn would lead to hypotriglyceridemia or hypertriglyceridemia. During fed-fast cycle, feeding could induce the expression of ANGPTL8 and activate the interaction between ANGPTL8 and ANGPTL3 in mice, thereby inhibiting LPL activity in the myocardium and bones. Conversely, fasting could inhibit the expression of ANGPTL8 and improve the expression of ANGPTL4 in mice, resulting the transportation of TG to muscles [20].
So far, studies on the ANGPTLs have focused on mice and humans [21], but reports on the ANGPTL gene in domestic animals are limited. The Jinhua pig is an important breed in China with a high percentage of body fat and good meat quality. Pigs are more closely related to humans in carbohydrate and lipid metabolism than mice [22]. Compared to mice, pigs are considered to be more suitable to be developed as animal models for studying human obesity and fat metabolism [23]. In this study, we reported the basic information of the ANGPTL1–8 gene in Jinhua pigs, the evolutionary relationship between the pig ANGPTL1–8 gene and other different vertebrates, their expression profile in different tissues, and the differences in the mRNA expression levels of ANGPTL3, ANGPTL4, and ANGPTL8 between obese and lean Jinhua pigs. The purpose of this study is to establish a theoretical foundation for the roles of the ANGPTL family genes in the fat regulation of pigs.
All animals used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of Zhejiang Academy of Agricultural Sciences (2019ZAASLA37). Written informed consent was obtained from the owners for the participation of their animals in this study.
The genome portion of pig ANGPTL family members were retrieved from the NCBI database to obtain basic information, such as transcript ID and mRNA length (bp) [24]. The relative molecular weights and charge was performed using the programs within the DNASTAR Lasergene [25], the ClustalW was used to generate the amino acid sequences [26]. Exon-intron boundaries and chromosomal locations were identified by the mRNA-genome alignment program Spidey [27].
The MUSCLE program was used to align amino acid sequences (obtained from the NCBI database), and Boxhade software was used for visual display. Phylogenetic analysis of the ANGPTL family members in humans and animals, including mice, cattle, pigs, dogs, and chickens, was carried out using the deduced mature protein sequences. The Mega7.0 software was used to construct phylogenetic trees from P-distances of ANGPTL amino acid sequences from pigs and other representative vertebrates [28]. The Bootstrap method was used to test the reliability of each branch 1,000 times.
In order to further confirm the homology of the ANGPTL gene and establish the evolutionary model of the ANGPTL gene in metazoa, we isolated the genetic environment of ANGPTL3 and ANGPTL4 chromosomes or genomic fragments of vertebrates to determine whether there are homologous genomic regions in vertebrates. Similarly, the genetic environment of mammalian ANGPTL8 is described. The short-range gene linkage comparison was performed among humans, mice, cattle, pigs, dogs, and poultry. The adjacent genetic environment of vertebrates was retrieved from the Genomicus database, and the genetic environment of human ANGPTL3, ANGPTL4, and ANGPTL8 were used as a reference sequence [29]. Homologous genomic regions were characterized by the discovery of specific genomic combinations of conserved flanking genes.
A total of 60 Jinhua Pigs at 90-day old were obtained from the experimental pig farm of Jinhua Academy of Agricultural Sciences (Jinhua, Zhejiang Province, China). All Jinhua pigs were reared in pens (10 pens, 6 pigs per pen) in an environmentally controlled facility and had free access to commercial diets and water under a standard management. At 270 days of age, pigs were weighed individually and slaughtered. Backfat thickness was measured. Based on the body weight data, different tissues were collected from 6 pigs with average body weight, immediately frozen in liquid nitrogen, and stored in a −80°C freezer until RNA isolation. The tissues included tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, trachea, lung, heart, longissimus muscle, subcutaneous fat, liver, kidney, brain, pancreas, and spleen. Eight of 92.35±10.10 kg pigs with the higher and lower backfat thickness were set as the high group (H group) and low group (L group), respectively [30]. The ileum, subcutaneous fat, and liver segments of the H and L group pigs were collected, immediately frozen in liquid nitrogen, and stored in a −80°C freezer until RNA isolation.
According to the manufacturer’s instructions, total RNA was extracted from each tissue sample using a TRI-zol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA, USA). RNA integrity and purity were assessed by Nanodrop after being electrophoresed in a formaldehyde gel [31,32]. The genomic DNA contamination was removed from the RNA samples using a gDNA Eraser (Takara Bio Inc., Dalian, China), and the PrimeScript RT reagent kit (Takara Bio Inc., China) was subsequently used according to the manufacturer’s instructions for reverse transcription. The cDNA was then diluted ten times with RNase-free water before quantitative real-time quantitative polymerase chain reaction (RT-qPCR) analysis. RT-qPCR was performed on each sample by using the CFX96 RT-qPCR Detection System (Bio-Rad Laboratories, Richmond, VA, USA), and the amplification was conducted in a total volume of 20 μL, containing 10 μL of SYBR Premix Ex Taq II (Takara Bio Inc., China), 7 μL of RNase-free water, 1 μL of the diluted cDNA, and 0.5 μL of each primer (Table 1). The optimum RT-qPCR program was 95°C for 1 min, followed by 40 cycles of 95°C for 15 s and 60°C for 25 s. Relative quantification of the mRNA transcripts was accomplished using the 2-ΔΔCT method and the housekeeping gene was glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Data analysis were performed by SPSS 23.0 (IBM, New York, NY, USA) followed by an unpaired two-tailed Student’s t-test [33]. Relative expression data are presented as mean±standard error of the mean. Statistical significance was considered at p<0.05. The relative expression levels of ANGPTL3, ANGPTL4, and ANGPTL8 in different tissues from Jinhua Pigs with different amounts of body fat were performed with the GraphPad Prism version 8.00 (GraphPad Software, LaJolla, CA, USA).
The nucleotide sequences of pig ANGPTL1–8 were obtained from the NCBI database, and the basic information was generated by DNASTAR Lasergene (Table 1). The mRNA sequences of pig ANGPTL1–8 were between 1,100 and 2,000 bp in length; the numbers of amino acid residues of ANGPTL1–7 were between 344–493 while ANGPTL8 were198. ANGPTL1–8 was composed of 4 to 8 exons determined by Spidy program analysis (Table 2).
Multiple sequence alignment of amino acid sequences of pig ANGPTL1–8 showed that the amino acid sequence of ANGPTL1–7 was highly conserved, and the amino acid sequence of ANGPTL8 was less conserved (Figure 1). Therefore, the structure of ANGPTL8 may be different from other members in the ANGPTL family. According to the Editsequence calculation, amino acid sequences of pig ANGPTL1–8 had 95.93%, 97.57%, 84.13%, 79.37%, 91.75%, 88.35%, 89.28%, and 71.72% homology with the corresponding amino acid sequences of human ANGPTL1–8.
Phylogenetic analysis of the vertebrate ANGPTL family suggested that these genes shared a common origin (Figure 2). According to the dendritic topology, there are five main protein clusters that contain different members of the ANGPTL family: the ANGPTL1-2-6 cluster (ANGPTL1, ANGPTL2, and ANGPTL6), the ANGPTL3–4 cluster (ANGPTL3, ANGPTL4), the ANGPTL5 cluster, the ANGPTL7 cluster, and the ANGPTL8 cluster, which was isolated from the other four protein clusters (Figure 2). Each ANGPTL orthologous gene was clustered with other mammalian sequences and separately from poultry.
By analyzing and comparing the genetic environment of ANGPTL3 (Figure 3) and ANGPTL4 (Figure 4) in mammals and poultry, we could further understand the evolutionary relationship of ANGPTL family genes in vertebrate radiation. The pig ANGPTL3 gene was mapped to chromosome 6, and the ANGPTL4 gene was mapped to chromosome 2 and was also found to be present in other mammals and chickens. Among the ten genes flanking human ANGPTL3, nine were retained on pig chromosome 6, and seven were retained on chicken chromosome 8. Of the ten genes flanking human ANGPTL4, nine were retained on pig chromosome 2, and nine were retained on chicken chromosome 28. This indicated that the genetic environment of several major mammals (humans, mice, cattle, pigs, and dogs) ANGPTL3 was highly similar and was also similar to that of chicken ANGPTL3, indicating that mammals and poultry ANGPTL3 and ANGPTL4 shared a common ancestral origin. Comparing the adjacent genetic environment of mammalian ANGPTL8 (Figure 5) in pigs, ANGPTL8 was located on chromosome 2. Among the ten genes flanking human ANGPTL8, all were retained on cow chromosome 7, and nine genes were retained on pig chromosome 2. This indicated that the genetic environment of ANGPTL8 in several major mammals (humans, mice, cattle, pigs, dogs) were highly similar, which means that the mammalian ANGPTL8 had a common ancestral origin.
In order to study the expression patterns of the ANGPTL gene family in pigs, the mRNA abundance of ANGPTL1–8 in 18 tissues of 6 Jinhua pigs was determined by RT-qPCR. As shown in Figure 6, ANGPTL4 was widely expressed in various tissues, while ANGPTL3 and ANGPTL8 were predominantly expressed in the liver, and ANGPTL8 was only expressed in the liver. Meanwhile, it was observed that ANGPTL4 was highly expressed in the intestinal tissues with relative low expression in subcutaneous fat, lung, heart, and longissimus muscle. ANGPTL4 relative expression level was the highest in subcutaneous fat.
To confirm whether lipid metabolism was associated with the expression of ANGPTL3, ANGPTL4, and ANGPTL8, eight of 92.35±10.10 kg pigs with the higher and lower backfat thickness were set as the high group (H group) and low group (L group). Pig ANGPTL3, ANGPTL4, and ANGPTL8 were analyzed by RT-qPCR following RNA isolation and reverse transcription. The backfat thickness of Jinhua pigs in the H group was significantly higher than that of Jinhua pigs in the L group (Figure 7). However, there were no significant differences in the body weight of Jinhua pigs between the H and L groups (Figure 7). The relative expression of ANGPTL3 in the liver of the H group pigs was significantly lower than that of the L group pigs (p = 0.005) while the relative expression levels of ANGPTL4 in the ileum (p = 0.043), subcutaneous fat (p = 0.025), and liver (p = 0.008) of the H group pigs were lower than those of the L group pigs (Figure 8). However, the relative expression of ANGPTL8 in the liver of the H group pigs was not significantly different from that of the L group pigs (p>0.05).
The Jinhua pig has become one of the most important local breeds in China due to its excellent meat quality and it exhibits the characteristics of early sexual maturity, low prolificacy, and a high body fat content [34]. The purpose of this study was to identify the phylogenetic and expression analysis of ANGPTL family genes in Jinhua Pig, a model animal of lipid metabolism, and the important roles of ANGPTL3, ANGPTL4, and ANGPTL8 in lipid metabolism. In the present study, amino acid sequence analysis, phylogenetic analysis, chromosome adjacent gene analysis, and RT-qPCR analysis showed that Jinhua pig ANGPTL family members had common characteristics with homologous human genes, including the high similarity of amino acid sequence and chromosome flanking genes. Consistent with earlier studies, pig ANGPTL3–4 had typical angiopoietin family structures. The structures of pig ANGPTL3–4 is similar to those of humans and mice, and both had FReD, which affects angiogenic activity, and CCD, which regulated fat metabolism by inhibiting LPL [35,36]. The eight ANGPTL family member genes in Jinhua pigs were 1,100 to 2,000 bp in length and consisted of 4 to 8 exons. The functional helical domain and fibrinogen-like domain in the ANGPTL1–8 amino acid sequence were strongly conserved. Through the identification and expression analysis of the bovine ANGPTL1–7 genes, all of the deduced amino acid sequences had an N-terminal crimped domain and a C-terminal fibrinogen-like domain, and both had the characteristics of angiogenin. The bovine ANGPTL1–7 amino acid sequences are similar to those of humans, and the corresponding members had 95%, 97%, 82%, 85%, 87%, 85%, and 87% homology [27]. Related studies reported the cloning, chromosomal location, and expression analysis of pig ANGPTL3 and ANGPTL4. The deduced amino acid sequence of pig ANGPTL3 had 83% and 73.7% homology to humans and mice, respectively. While pig ANGPTL4 had 79.4% and 79.1% homology with humans and mice [36]. Homologs of the mammalian ANGPTL8 gene are not present in sequenced genomes of fish and other non-mammalian vertebrates, ANGPTL8 is an atypical family member that lacks the FReD structural domain, glycosylation sites and amino acids that form intramolecular disulfide bonds, leading to a lower homology of ANGPTL8 with humans in other species [37]. This research showed that pig ANGPTL1–8 amino acid sequence had 95.93%, 97.57%, 84.13%, 79.37%, 91.75%, 88.35%, 89.28%, and 71.72% homology with corresponding human members, respectively.
In vertebrates, the ANGPTL family of genes evolved by gene duplication, gene deletion, and gene mutation [37]. They share the same evolutionary origin with angiogenin and share similarities in sequence and structure [37]. ANGPTL family members expanded in early vertebrate genome doubling and genome segment replication before vertebrate radiation. Five major ANGPTL vertebrate protein clusters, ANGPTL1-2-6, ANGPTL3–4, ANGPTL5, ANGPTL7, ANGPTL8, derived from the replication of ancestor ANGPTL [38]. The ANGPTL8 cluster was isolated from the other four protein clusters since ANGPTL8 has a different structure from other ANGPTL family members [19]. Clustering in phylogenetic trees indicates that pig sequences are not significantly different from those of other vertebrates.
Recent studies have shown that ANGPTL3, ANGPTL4, and ANGPTL8 are directly related to lipid metabolism and fat deposition in human and mice [39]. In this study, tissue expression profiles showed that ANGPTL3 and ANGPTL8 were mainly expressed in the liver of pigs, with similar results in humans and mice [5,40]. However, ANGPTL4 in pigs is widely expressed in many tissues, including the gastrointestinal tract, subcutaneous fat, liver, muscles, heart, and lungs. In humans and mice, ANGPTL4 is also expressed mainly in white adipose tissue and the liver as well as in the intestine and heart tissue [10,41]. By analyzing the expression levels of ANGPTL3 and ANGPTL8 in the liver and the relative expression levels of ANGPTL4 in the liver, subcutaneous fat, and ileum of Jinhua pigs with distinct fatness, we found that the expression of ANGPTL3 in the liver of Jinhua pigs in the H group was significantly lower than that of Jinhua pigs in the L group (p = 0.005). Moreover, the expression level of ANGPTL4 in the liver (p = 0.008), subcutaneous fat (p = 0.025), and ileum (p = 0.043) of the H group pigs was significantly lower than that of the L group pigs. This might be due to the inhibitory effect of ANGPTL3 and ANGPTL4 on LPL. With the decrease in the expressions of ANGPTL3 and ANGPTL4, the activity of LPL would increase, which in turn would decrease the serum TG level and increase the lipid absorption. Early research showed that in sterile mice with routine feeding, the microbiota promoted the storage of triglycerides in fat cells by inhibiting the intestinal expression of ANGPTL4, illustrating the decrease of ANGPTL4 expression is beneficial to fat storage [11]. Recent studies have indicated that the level of circulating ANGPTL4 in obese children was lower than that of normal weight children. Waist circumference, body weight, and other major obesity indicators were negatively correlated with the level of circulating ANGPTL4 [42]. Meanwhile, mice with ANGPTL3 and ANGPTL4 deficiency had severe hypertriglyceridemia while mice with ANGPTL3 overexpression had hyperlipidemia [43]. Furthermore, compared with other breeds of pigs, the fat percentage of Large White pigs was lower with a higher expression of ANGPTL4 mRNA [30]. These studies are consistent with the results of the present study.
Although there was no significant difference in ANGPTL8 expression between the liver of Jinhua pigs in the H and L groups, it might result from the function of ANGPTL8, which promotes the cleavage of ANGPTL3 and binds to the N-terminal of ANGPTL3 [20]. Related studies have confirmed that expression of ANGPTL8 in ANGPTL3 knockout mice failed to promote hypertriglyceridemia [19]. Additionally, the overexpression of ANGPTL8 in the liver of ANPTL3 gene knockout mice had no effect on the triglyceride metabolism [19]. The interaction of ANGPTL8 with ANGPTL3 would form a complex with the N-terminal of ANGPTL3, synergistically inhibiting the LPL activity and modulating the plasma triglyceride levels [44].
Fat metabolism and fat storage are closely related to the meat quality and carcass quality. Relevant research showed that by comparing the carcass composition and development patterns of Jinhua and Landrace pigs at 35 to 125 days of age, the carcass fat content of Jinhua pigs was significantly higher than that of Landrace pigs (p<0.05), and the lean meat percentage of the p carcass was significantly lower than that of Landrace pigs (p<0.01) [45]. Similar studies showed that Jinhua pigs had a higher tendency to deposit fat compared with Landrace pigs [23]. These are consistent with the results of this experiment. Therefore, Jinhua Pigs with high body fat percentage and good meat quality play an important role in the study of lipid metabolism and its relationship with meat quality.
Overall, the present study identified the basic information and phylogenetic patterns of porcine ANGPTL family gene members, and the expression of ANGPTL1–8 in Jinhua pigs was tissue-specific. This study enriches our understanding of the porcine ANGPTL family genes and confirm the important relevance of ANGPTL3 and ANGPTL4 in regulating lipid metabolism and improving pork quality, heralding a new research direction and molecular theoretical basis for improving pork quality.
Notes
AUTHOR CONTRIBUTIONS
ZZ, WL, YR, and YX designed the experiment. ZZ, WL, QH, HY, YR, and YX conducted the animal experiments. ZZ, WL, HY, YR, and YX wrote and revised the manuscript. ZZ, WL, YR, HY, and YX did the experimental analysis, collected, and analyzed the data. All authors reviewed the manuscript.
AVAILABILITY OF DATA AND MATERIALS
The datasets reported in this manuscript are available from the corresponding author on reasonable request. The genome portion of pig ANGPTL family members were retrieved from the NCBI database to obtain basic information. The MUSCLE program was used to align amino acid sequences (obtained from the NCBI database), and Boxhade software was used for visual display. The ANGPTL1–8 gene sequence was obtained in NCBI, and their genebank accession numbers are NM_001109947.1, NM_001109946.1, NM_00100 3926.2, NM_001038644.1, XM_003129804.4, XM_00312 3226.5, NM_001142828.2, and XM_021083871.1 respectively.
Figure 1
Multiple sequence alignment of representative pig angiopoietin-like (ANGPTL) protein sequences. The conserved residues are shaded. Specifically, the black part is highly similar, the gray part is less similar, and the non-color covered part has no similarity.
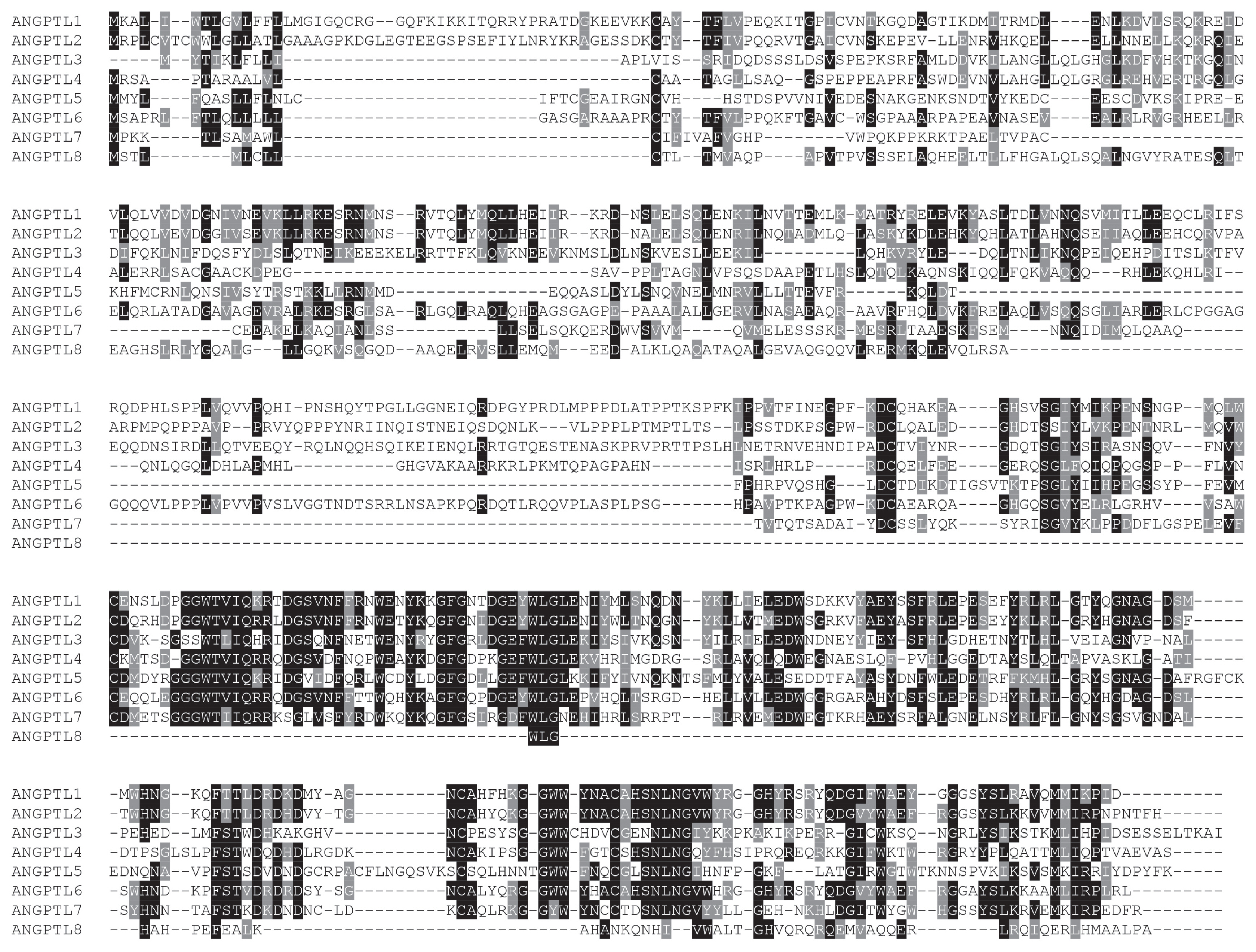
Figure 2
Phylogenetic analysis of pig angiopoietin-like (ANGPTL) genes and their representative sequences from human and animals. The topology was constructed using the amino acid sequences by the Neighbor-Joining method with 1,000 bootstrap repeats. Only the branches with a bootstrap value greater than 50 are displayed at branching points.
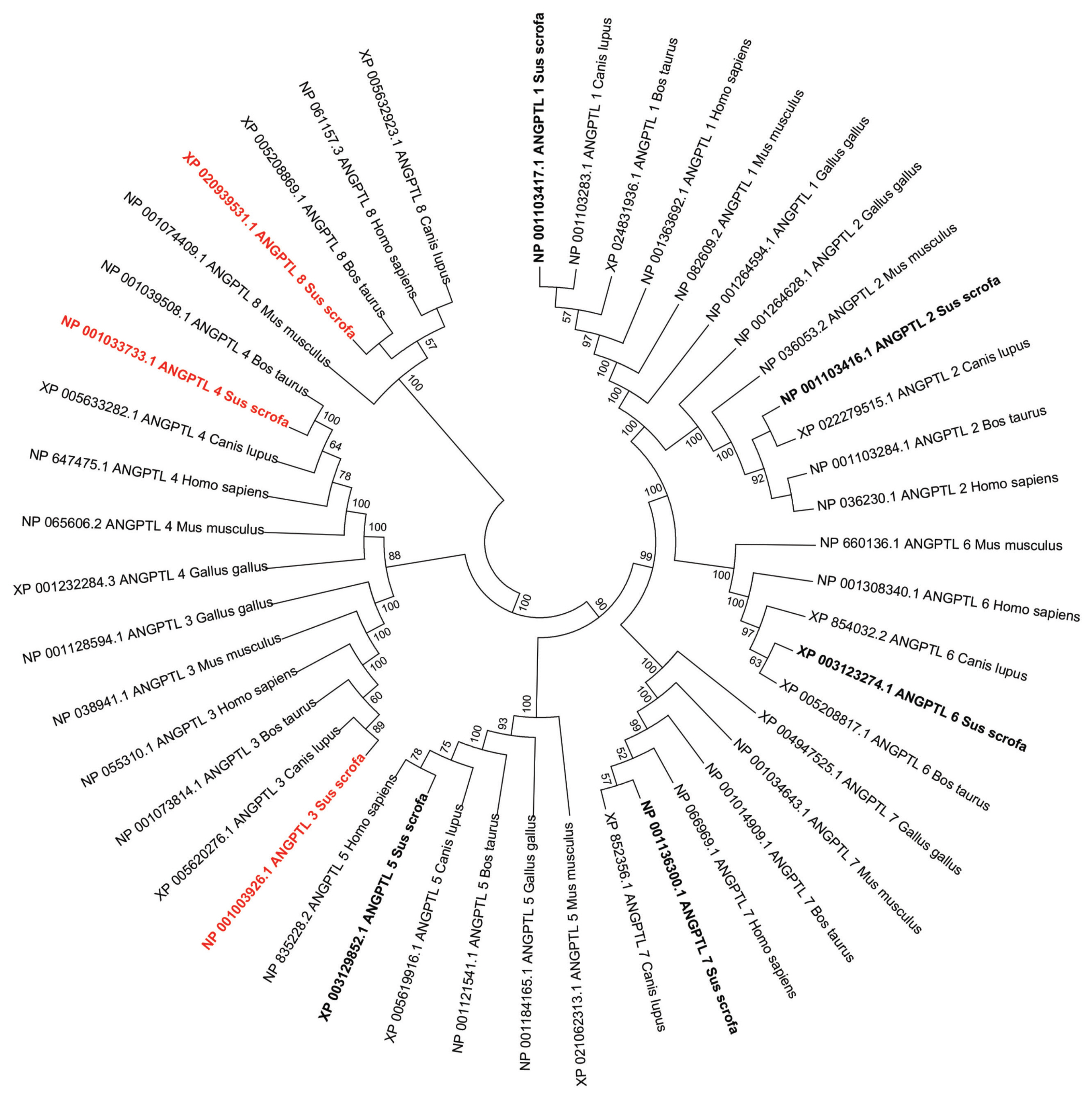
Figure 3
Comparison of homologous genomic regions of pig ANGPTL3 with humans, mice, and several other animals. The genetic environment of the pig ANGPTL3 gene was characterized, and the homologous genes of humans, mice, and other animals were identified. The horizontal line represents the chromosome fragments, the arrow box represents the gene, and the arrowhead points in the direction of predicted gene transcription. Only genes conserved across species are displayed. The same color is of the gene homologs, and they are presented according to their order in the chromosome. The gene names and symbols are: PATJ, PATJ crumbs cell polarity complex component); L1TD1, LINE1 type transposase domain containing 1; KANK4, KN motif and ankyrin repeat domains 4; USP1, ubiquitin-specific peptidase 1; DOCK6, dedicator of cytokinesis 6; ANGPTL3, angiopoietin-like 3; ATG4C, autophagy related 4C cysteine peptidase; FOXD3, forkhead box D3; ALG6, ALG6 alpha-1,3-glucosyltransferase; ITGB3BP, integrin subunit beta 3 binding protein; EFCAB7, EF-hand calcium binding domain 7.
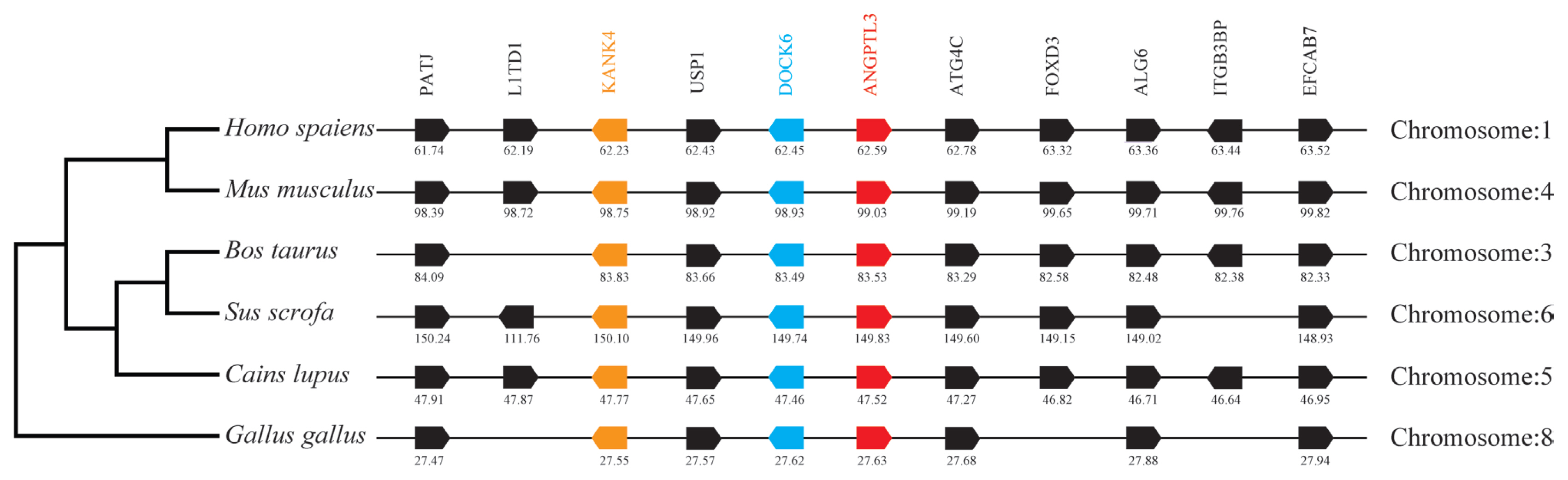
Figure 4
Comparison of homologous genomic regions of pig ANGPTL4 with humans, mice, and several other animals. The genetic environment of the pig ANGPTL4 gene was characterized, and the homologous genes of humans, mice, and other animals were identified. The horizontal line represents the chromosome segment, the arrow box represents the gene, and the arrowhead points in the direction of predicted gene transcription. Only genes preserved across species will appear. The same color is of the gene homologs, and they are presented according to their order in the chromosome. The gene names and symbols are: CERS4, ceramide synthase 4; CD320, CD320 molecule; DUFA7, NADH:ubiquinone oxidoreductase subunit A7; RPS28, Ribosomal protein S28; KANK3, KN motif, and ankyrin repeat domains 3; ANGPTL4, angiopoietin-like 4; Rab11B, RAB11B, member RAS oncogene family; MARCH2, membrane-associated ring finger (C3HC4) 2; HNRNPM, heterogeneous nuclear ribonucleoprotein M; PRAM1, PML-RARA regulated adaptor molecule 1; ZNF414, zinc finger protein 414.
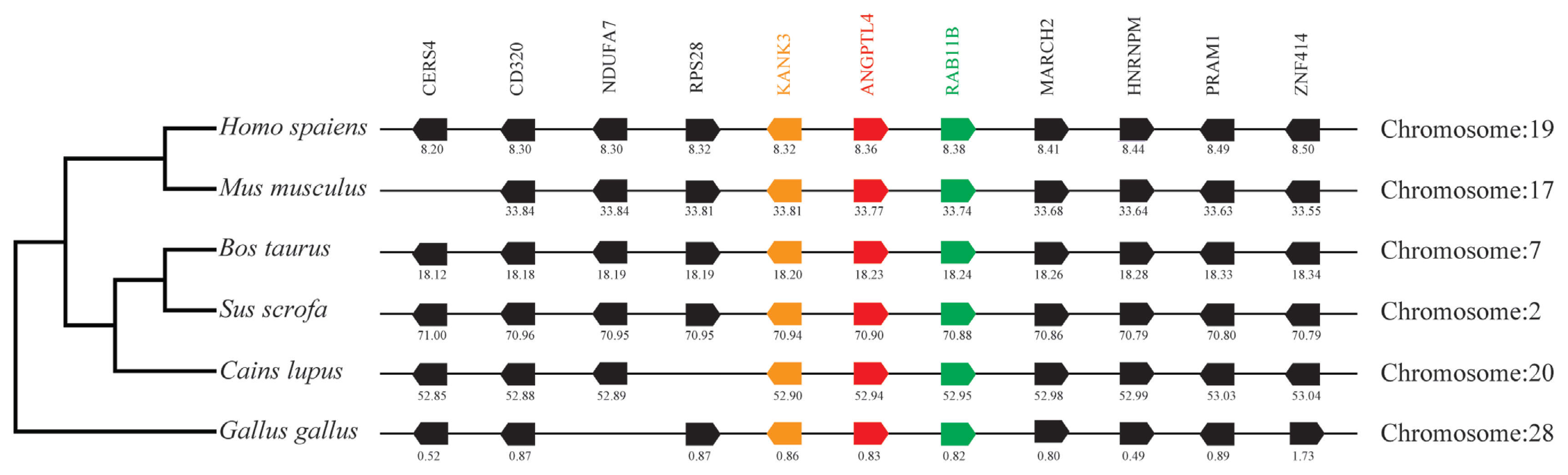
Figure 5
Comparison of homologous genomic regions of pig ANGPTL8 with humans, mice, and several other animals. The genetic environment of the pig ANGPTL8 gene was characterized, and the homologous genes of humans, mice, and other animals were identified. The horizontal line represents the chromosome segment, the arrow box represents the gene, and the arrowhead points in the direction of predicted gene transcription. Only genes preserved across species will appear. The same color is of the gene homologs, and they are presented according to their order in the chromosome. The gene names and symbols are: SMARCA4, SWI/SNF related, matrix associated, actin-dependent regulator of chromatin, subfamily a, member 4; LDLR, low density lipoprotein receptor; SPC24, SPC24 component of NDC80 kinetochore complex; KANK2, KN motif and ankyrin repeat domains 2; DOCK6, dedicator of cytokinesis 6; ANGPTL8, angiopoietin-like 8; SPAN16, sperm peptide antigen; RAB3D, RAB3D, member RAS oncogene family; TMEM250, Transmembrane protein 250; CCDC159, Coiled-coil domain containing 159; PLPPR2, phospholipid phosphatase related 2.
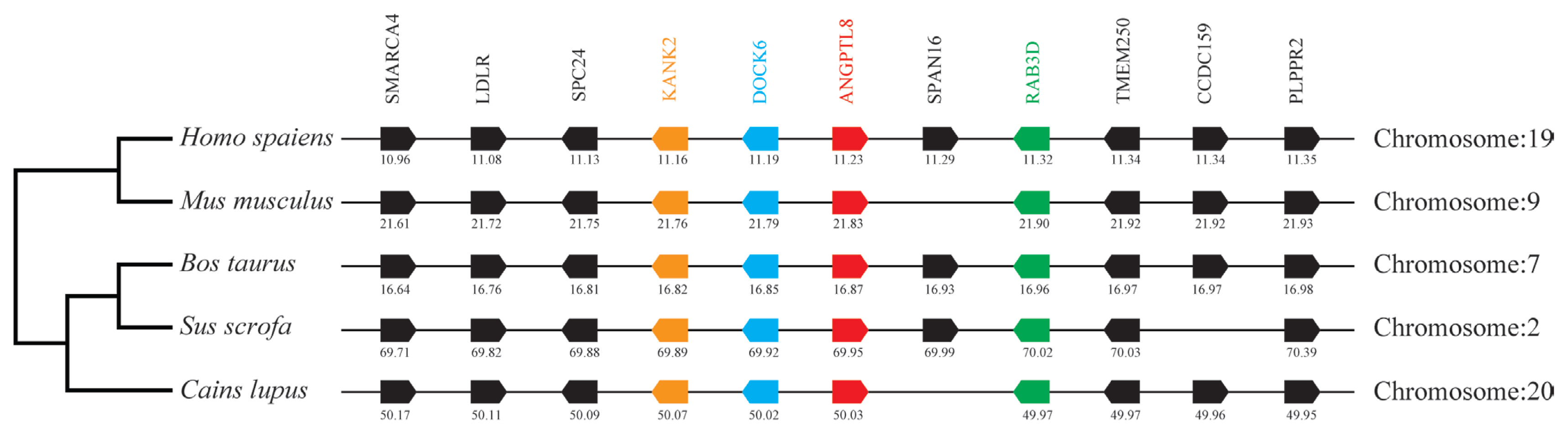
Figure 6
Relative expression of ANGPTL1–8 in different tissues, including tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, trachea, lung, heart, longissimus muscle, subcutaneous fat, liver, kidney, brain, pancreas, and spleen of Jinhua pigs. The indicated tissue segments were collected from 6 Jinhua pigs at 270 days of age following by RNA isolation, reverse transcription and RT-qPCR. ANGPTL8, angiopoietin-like 8. Data was expressed as mean±standard error of the mean (n = 6).
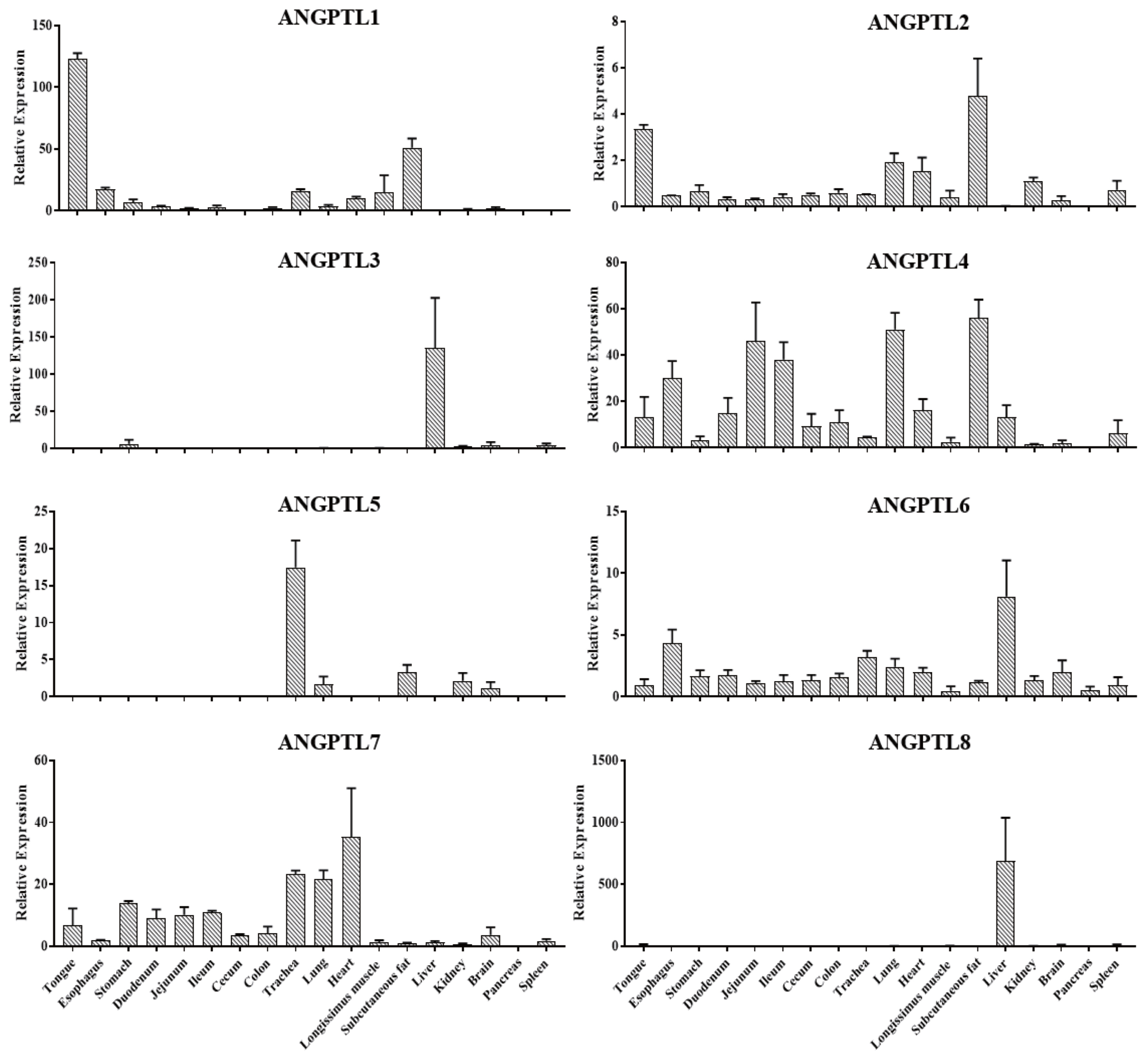
Figure 7
Body weight (A) and backfat thickness (B) of Jinhua pigs in the H and L groups. Jinhua pigs with similar body weight and significantly different backfat thickness were weighed individually and the backfat thickness of each pig was measured at 270 days of age. H, the H group consisted of pigs with relatively high backfat thickness; L, the L group was consisted of pigs with relatively low backfat thickness. ANGPTL, angiopoietin-like. Data was expressed as mean±standard error of the mean (n = 8) and analyzed by one-way analysis of variance analysis followed by an unpaired two-tailed student’s t-test.

Figure 8
The expression of ANGPTL3, ANGPTL4, and ANGPTL8 in the liver (A, B, and C), subcutaneous fat (D), and ileum (E) of Jinhua pigs in the H and L groups. The liver, subcutaneous fat, and ileum segments were collected from 16 Jinhua pigs at 270 days of age following by RNA isolation, reverse transcription and RT-qPCR. H, the H group consisted of pigs with relatively high backfat thickness; L, the L group was consisted of pigs with relatively low backfat thickness. Data was expressed as mean±standard error of the mean (n = 8) and analyzed by one-way analysis of variance analysis followed by an unpaired two-tailed student’s t-test.
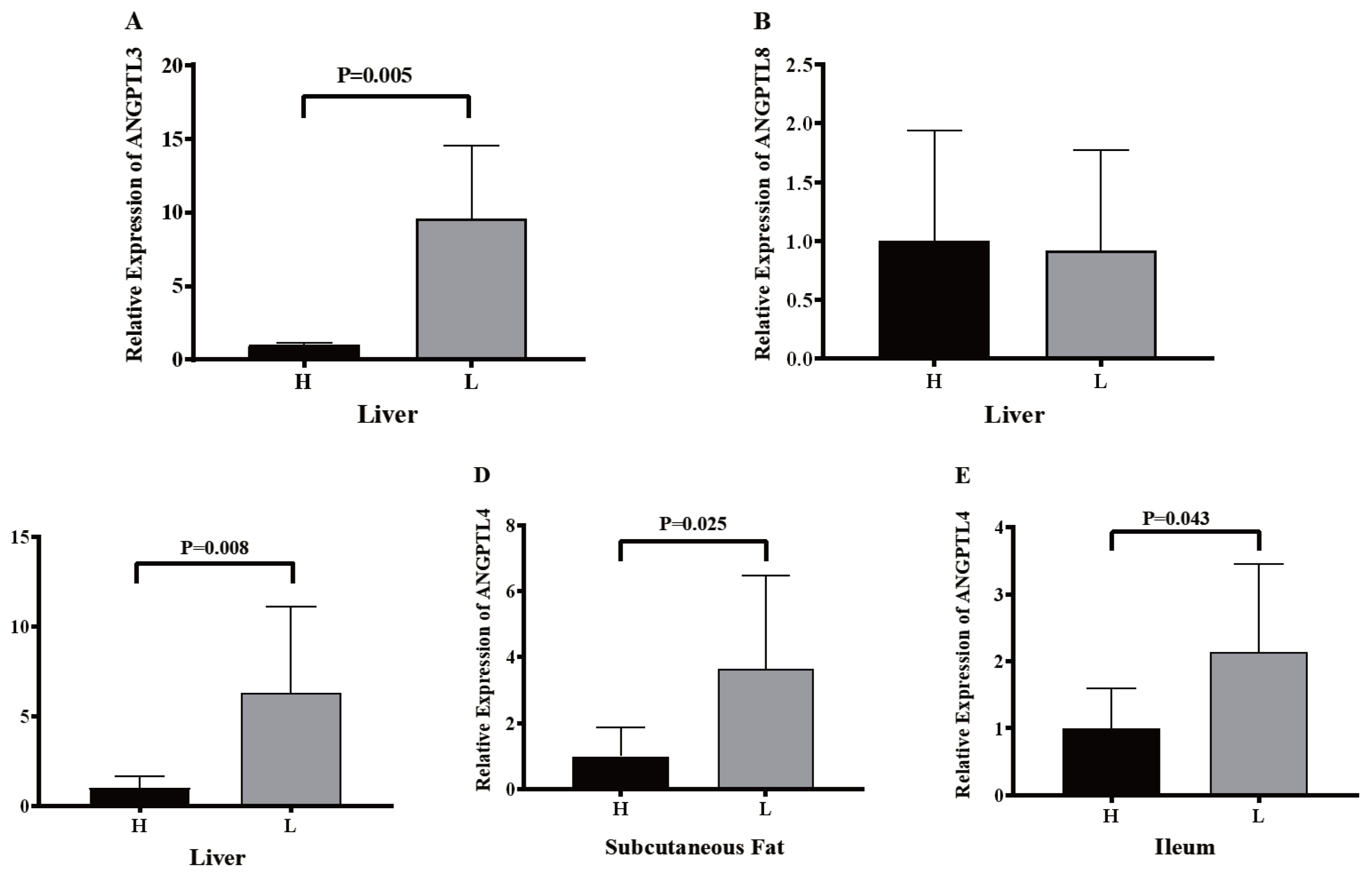
Table 1
Primers sequences used in the RT-qPCR analysis
Table 2
Basic information of pig ANGPTL1–8
REFERENCES
1. Abu-Farha M, Ghosh A, Al-Khairi I, Murthy Madiraju SR, Abubaker J, Prentki M. The multi-faces of Angptl8 in health and disease: Novel functions beyond lipoprotein lipase modulation. Prog Lipid Res 2020; 80:101067
https://doi.org/10.1016/j.plipres.2020.101067


2. Zhang R, Abou-Samra AB. Emerging roles of Lipasin as a critical lipid regulator. Biochem Biophys Res Commun 2013; 432:401–5.
https://doi.org/10.1016/j.bbrc.2013.01.129


3. Morelli MB, Chavez C, Santulli G. Angiopoietin-like proteins as therapeutic targets for cardiovascular disease: focus on lipid disorders. Expert Opin Ther Targets 2020; 24:79–88.
https://doi.org/10.1080/14728222.2020.1707806



4. Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology 2021; 160:573–99.
https://doi.org/10.1053/j.gastro.2020.10.057


5. Li J, Li L, Guo D, et al. Triglyceride metabolism and angiopoietin-like proteins in lipoprotein lipase regulation. Clin Chim Acta 2020; 503:19–34.
https://doi.org/10.1016/j.cca.2019.12.029


6. Davies BS, Beigneux AP, Barnes RH, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab 2010; 12:42–52.
https://doi.org/10.1016/j.cmet.2010.04.016



7. Mohamed F, Mansfield BS, Raal FJ. ANGPTL3 as a drug target in hyperlipidemia and atherosclerosis. Curr Atheroscler Rep 2022; 24:959–67.
https://doi.org/10.1007/s11883-022-01071-1



8. Kersten S. Angiopoietin-like 3 in lipoprotein metabolism. Nat Rev Endocrinol 2017; 13:731–9.
https://doi.org/10.1038/nrendo.2017.119


9. Wang Y, McNutt MC, Banfi S, Hobbs HH. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc Natl Acad Sci USA 2015; 112:11630–5.
https://doi.org/10.1073/pnas.1515374112



10. Ruppert PMM, Michielsen C, Hazebroek EJ, et al. Fasting induces ANGPTL4 and reduces LPL activity in human adipose tissue. Mol Metab 2020; 40:101033
https://doi.org/10.1016/j.molmet.2020.101033



11. Bäckhed F, Ding H, Wang T, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004; 101:15718–23.
https://doi.org/10.1073/pnas.0407076101



12. Alex S, Lichtenstein L, Dijk W, et al. ANGPTL4 is produced by entero-endocrine cells in the human intestinal tract. Histochem Cell Biol 2014; 141:383–91.
https://doi.org/10.1007/s00418-013-1157-y


13. Ruscica M, Zimetti F, Adorni MP, et al. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol Res 2020; 153:104653
https://doi.org/10.1016/j.phrs.2020.104653


14. Mattijssen F, Alex S, Swarts HJ, Groen AK, van Schothorst EM, Kersten S. Angptl4 serves as an endogenous inhibitor of intestinal lipid digestion. Mol Metab 2014; 3:135–44.
https://doi.org/10.1016/j.molmet.2013.11.004



15. Dijk W, Kersten S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr Opin Lipidol 2016; 27:249–56.
https://doi.org/10.1097/MOL.0000000000000290


16. Chen YQ, Pottanat TG, Siegel RW, et al. Angiopoietin-like protein 4(E40K) and ANGPTL4/8 complex have reduced, temperature-dependent LPL-inhibitory activity compared to ANGPTL4. Biochem Biophys Res Commun 2021; 534:498–503.
https://doi.org/10.1016/j.bbrc.2020.11.053


17. Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun 2012; 424:786–92.
https://doi.org/10.1016/j.bbrc.2012.07.038


18. Chi X, Britt EC, Shows HW, et al. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab 2017; 6:1137–49.
https://doi.org/10.1016/j.molmet.2017.06.014



19. Quagliarini F, Wang Y, Kozlitina J, Hobbs HH. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci USA 2012; 109:19751–6.
https://doi.org/10.1073/pnas.1217552109



20. Zhang R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol 2016; 6:150272
https://doi.org/10.1098/rsob.150272



21. Liu YZ, Zhang C, Jiang JF, et al. Angiopoietin-like proteins in atherosclerosis. Clin Chim Acta 2021; 521:19–24.
https://doi.org/10.1016/j.cca.2021.06.024


22. Poledne R, Jurčíková-Novotná L. Experimental models of hyperlipoproteinemia and atherosclerosis. Physiol Res 2017; 66:Suppl 1S69–s75.
https://doi.org/10.33549/physiolres.933585


23. Xiao Y, Kong F, Xiang Y, et al. Comparative biogeography of the gut microbiome between Jinhua and Landrace pigs. Sci Rep 2018; 8:5985
https://doi.org/10.1038/s41598-018-24289-z



24. Geer LY, Marchler-Bauer A, Geer RC, et al. The NCBI BioSystems database. Nucleic Acids Res 2010; 38:Suppl 1D492–6.
https://doi.org/10.1093/nar/gkp858



25. Blanton LS, Quade BR, Bouyer DH. Differentiation of rickettsia felis and rickettsia felis-like organisms via restriction fragment length polymorphism analysis. Vector Borne Zoonotic Dis 2019; 19:637–9.
https://doi.org/10.1089/vbz.2018.2428



26. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22:4673–80.
https://doi.org/10.1093/nar/22.22.4673



27. Guan Z, Cai G, Sun J, Lu J. Identification and expression analysis of bovine ANGPTL gene family. Curr Zool 2010; 56:445–53.
https://doi.org/10.1093/czoolo/56.4.445

28. Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870–4.
https://doi.org/10.1093/molbev/msw054



29. Louis A, Nguyen NT, Muffato M, Crollius HR. Genomicus update 2015: KaryoView and MatrixView provide a genome-wide perspective to multispecies comparative genomics. Nucleic Acids Res 2015; 43:D682–9.
https://doi.org/10.1093/nar/gku1112



30. Yang H, Huang X, Fang S, Xin W, Huang L, Chen C. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci Rep 2016; 6:27427
https://doi.org/10.1038/srep27427



31. Jin L, Yu JP, Yang ZJ, et al. Modulation of gene expression in liver of hibernating asiatic toads (Bufo gargarizans). Int J Mol Sci 2018; 19:2363
https://doi.org/10.3390/ijms19082363



32. Yan H, Zhou P, Zhang Y, Zhang Z, Liu J, Zhang H. Short-chain fructo-oligosaccharides alleviates oxidized oil-induced intestinal dysfunction in piglets associated with the modulation of gut microbiota. J Funct Foods 2020; 64:103661
https://doi.org/10.1016/j.jff.2019.103661

33. Yang H, Xiang Y, Robinson K, et al. Gut microbiota is a major contributor to adiposity in pigs. Front Microbiol 2018; 9:3045
https://doi.org/10.3389/fmicb.2018.03045



34. Yang H, Xiao Y, Wang J, et al. Core gut microbiota in Jinhua pigs and its correlation with strain, farm and weaning age. J Microbiol 2018; 56:346–55.
https://doi.org/10.1007/s12275-018-7486-8


35. Ono M, Shimizugawa T, Shimamura M, et al. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J Biol Chem 2003; 278:41804–9.
https://doi.org/10.1074/jbc.M302861200


36. Feng SQ, Chen XD, Xia T, et al. Cloning, chromosome mapping and expression characteristics of porcine ANGPTL3 and -4. Cytogenet Genome Res 2006; 114:44–9.
https://doi.org/10.1159/000091927


37. Costa RA, Cardoso JC, Power DM. Evolution of the angiopoietin-like gene family in teleosts and their role in skin regeneration. BMC Evol Biol 2017; 17:14
https://doi.org/10.1186/s12862-016-0859-x



38. Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 2005; 3:e314
https://doi.org/10.1371/journal.pbio.0030314



39. Kristensen KK, Leth-Espensen KZ, Mertens HDT, Ploug M. Unfolding of monomeric lipoprotein lipase by ANGPTL4: Insight into the regulation of plasma triglyceride metabolism. Proc Natl Acad Sci USA 2020; 117:4337–46.
https://doi.org/10.1073/pnas.1920202117



40. Catalano-Iniesta L, Sánchez Robledo V, Iglesias-Osma MC, et al. Evidences for Expression and location of ANGPTL8 in human adipose tissue. J Clin Med 2020; 9:512
https://doi.org/10.3390/jcm9020512



41. Santulli G. Angiopoietin-like proteins: a comprehensive look. Front Endocrinol (Lausanne) 2014; 5:4
https://doi.org/10.3389/fendo.2014.00004



42. Barja-Fernández S, Folgueira C, Castelao C, et al. ANGPTL-4 is associated with obesity and lipid profile in children and adolescents. Nutrients 2019; 11:1340
https://doi.org/10.3390/nu11061340



43. Xiao HB, Wang JY, Sun ZL. ANGPTL3 is part of the machinery causing dyslipidemia majorily via LPL inhibition in mastitis mice. Exp Mol Pathol 2017; 103:242–8.
https://doi.org/10.1016/j.yexmp.2017.11.003


44. Chi X, Britt EC, Shows HW, et al. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab 2017; 6:1137–49.
https://doi.org/10.1016/j.molmet.2017.06.014



45. Miao ZG, Wang LJ, Xu ZR, Huang JF, Wang YR. Developmental changes of carcass composition, meat quality and organs in the Jinhua pig and Landrace. Animal 2009; 3:468–73.
https://doi.org/10.1017/S1751731108003613








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





