 |
 |
| Anim Biosci > Volume 36(9); 2023 > Article |
|
Abstract
Objective
This study was conducted to evaluate Raman spectroscopy technique as a non-invasive tool to predict meat quality traits on Braford longissimus thoracis et lumborum muscle.
Methods
Thirty samples of muscle from Braford steers were analyzed by classical meat quality techniques and by Raman spectroscopy with 785 nm laser excitation. Water holding capacity (WHC), intramuscular fat content (IMF), cooking loss (CL), and texture profile analysis recording hardness, cohesiveness, and chewiness were determined, along with fiber diameter and sarcomere length by scanning electron microscopy. Warner-Bratzler shear force (WBSF) analysis was used to differentiate tender and tough meat groups.
Results
Higher values of cohesiveness and CL, together with lower values of WHC, IMF, and shorter sarcomere were obtained for tender meat samples than for the tougher ones. Raman spectra analysis allows tender and tough sample differentiation. The correlation between the quality attributes predicted by Raman and the physical measurements resulted in values of R2 = 0.69 for hardness and 0,58 for WBSF. Pearsonâs correlation coefficient of hardness (r = 0.84) and WBSF (r = 0.79) parameters with the phenylalanine Raman signal at 1,003 cmâ1, suggests that the content of this amino acid could explain the differences between samples.
Tenderness and juiciness are the most important quality traits for overall beef palatability [1]. In addition, studies show that consumers are willing to pay a higher price for beef cuts with guaranteed tenderness [2]. The best approach to assessing sensory attributes that provide the most accurate prediction of customer response is through sensory panels. However, these tests are not suitable for routine application by commercial processors as they are destructive, resource-intensive, costly, and time-consuming. Therefore, it is necessary to develop rapid, non-invasive, and non-destructive methods for the evaluation of meat quality that are suitable for use by commercial meat processors [3]. In this regard, several studies have been conducted to investigate the correlation between these sensory properties and the physical parameters measured [4â6]. Methods based on early postmortem assessment of meat quality, such as DNA or protein analysis, are accurate but are limited by the need for expensive instruments and skilled personnel.
Recent technological advances have made optical and spectroscopic methods more suitable for the commercial evaluation of meat quality [7â10]. Among them, Raman spectroscopy is a vibrational spectroscopic method that can be used to evaluate both the structure and composition of meat samples [11]. Several studies have reported on the potential of this technique in combination with chemometric analysis to predict objective meat quality traits in pork [12,13], lamb [14,15], and beef [16â19]. Previously, it was suggested that some muscles from some species may have a better prediction of meat quality traits [20]. For instance, Bauer et al [17] predicted shear strength with R2 = 0.33 with a 671 nm handheld Raman system in gluteus medius muscles of young bulls of different origin (90% Simmental and 10% mixed origin) aged for 14 days, Fowler et al [16], suggest that a 671 nm handheld Raman spectroscopic device can predict sensory tenderness with R2 = 0.47 in longissimus lumborum muscles aged 3 and 21 days. Promising results are reported in bovine longissimus dorsi muscles using Raman spectroscopy with 780 and 785 nm laser lines. In addition, Cama-Moncunill et al [18] predicted Warner-Bratzler shear strength with R2 = 0.48 in frozen longissimus thoracis et lumborum muscle of crossbred bull and elite Irish bovine steers using a Raman spectrometer with a 780 nm laser, and Chen et al [19] demonstrated that the portable Raman device with 785 nm laser could predict Meulle-net-Owens razor shear with R2 = 0.81 and toughness with R2 = 0.82 in 2-day-aged Chinese yellow (Yanbian) bull longissimus dorsi muscles.
In Argentina, as a consequence of the expansion of agriculture, British breeds were replaced by other genotypes, such as composites between Bos indicus and Bos Taurus breeds. Among them, development of the Braford breed was improved by challenging cattle production systems to meet the demand for high-quality meat. Therefore, in this research, we explore the capability of Raman spectroscopy using a 785 nm laser to assess post-mortem meat quality traits of Braford cattle longissimus thoracis et lumborum muscle as a simple and accurate analytical prediction tool for the meat industry.
Animal handling and experimental procedures were followed according to the Animal Welfare Procedures Manual of the National Animal Health Service of Argentina (SENASA). Samples of the longissimus thoracis et lumborum muscles were obtained from thirty Braford (5/8 Hereford y 3/8 Brahman) steers. Animals were slaughtered when they were an average age of 26 months and 464±34 kg body weight. After slaughter and a 48-hour chilling period between 1°C and 5°C, the fillet block between the 9th and 13th rib of each left half-carcass was removed. The block was deboned, vacuum packed, and frozen at â18°C until experimental determinations. This methodology was used to carry out the analysis of the samples in a controlled and sequential way, avoiding multiple freeze-thaw cycles [3,8].
All steak sections were divided into 2.5 cm thick slices, and before measurements, the slices were thawed at 4°C for 24 h.
Intramuscular fat content (IMF) was determined according to official methods [21] in two samples of 5 g, trimmed of external fat.
Water holding capacity (WHC) was determined following the filter paper press methodology [22]. Briefly, this technique consists of compressing 0.30±0.02 g of sample, using a 90 mm diameter filter paper (Munktell Filter, Quality 1003) and measuring the wetted area. The WHC was expressed as the percentage of meat-free juice expelled (WHC = meat area/total area of infiltrated liquid Ă100). This procedure assumes that the area of the squeezed juice ring absorbed by the filter paper is related to the amount of meat-free water. Each sample was analyzed twice.
For cooking loss (CL) determinations, each steak was deboned, trimmed of fat and epimysium, weighed, and subjected to heat treatment with dry-heat cooking (oven temperature, 170°C; temperature of the thermal center of the samples, 71°C) [23]. The CL value was calculated as the percentage of weight loss relative to the initial weight of the sample, (equation 1), where sample a and sample b are the sample weight before and after the heat treatment. respectively.
Texture profile analysis (TPA) and Warner-Bratzler shear force (WBSF) determinations were carried out with 2.5 cm thick steaks of cooked meat. Once thawed, meat cuts were deboned, weighed, and placed in a pre-heated electric grill until they reached a final internal temperature of 71°C. Cooked steaks were weighed and cooled down to <10°C overnight. TPA measurements were obtained by cutting 1.3 cm diameter cores from each steak parallel to the muscle fiber orientation each of which was subjected to the action of the TA.XT Plus texturometer (Stable Micro Systems Ltd, Surrey, UK) using a 10 mm in diameter cylindrical probe. The samples were then placed under the probe which moved downward at a constant speed of 3.0 mm/s (pre-test), 1.0 mm/s (test), and 3.0 mm/s (post-test). When the probe first came in contact with the sample, the software automatically recorded the thickness of the sample. The probe continued down a preset percentage of the sample thickness (75%), returned to the initial point of contact with the sample, and paused for a period of 2 s before starting the second compression cycle. Through this analysis, the parameters of hardness, cohesiveness, and chewiness were determined. Hardness is the maximum resistance during the first compression of meat, representing the hardness of the sample in the first bite [24]. Cohesiveness is the ratio (dimensionless) between the positive force during the second compression cycle and that of the first one and represents the strength of the internal bonds, which maintains the structure of a sample [25]. Finally, springiness is the degree to which the meat returns to its original height after being compressed. Therefore, the chewiness is calculated as hardnessĂcohesivenessĂspringiness, which is the energy required to chew a solid sample to a steady state of swallowing [4]. TPA measurements were performed in at least 7 to 10 cores per sample.
Instrumental tenderness was measured using the WBSF test, assessing the resistance to shear force cut in cooked meat as described before [26]. Samples were obtained by cutting 1.3 cm diameter cores from each steak parallel to the muscle fiber orientation and sheared once across the middle (in the direction perpendicular to the fibers) using a 1.016 mm WB probe in a TA.XT Plus Texture Analyzer (Stable Micro Systems Ltd, Surrey, UK). The highest peak in Newton (maximum shear force) was measured in 7 to 10 cores per sample.
To further analysis, meat samples were grouped according to their hardness values obtained by the WBSF test, considering the shear force threshold of 58.8 N suggested by Shackelford et al [27], which proposed that meat samples with shear force values lower than threshold could be deemed tender and a sample was deemed tough if its WBSF value was 58.8 N or greater. In this sense, samples with WBSF values below threshold were grouped in the Tender group (mean 54.98 N) and samples with higher values in the Tougher group (mean 74,91 N). This threshold value was chosen previously as the margin between tender and tough because the regression of trained sensory panel tenderness ratings on shear force indicates that a sample with a shear value of 58.8 N will be rated âslightly tenderâ on average.
Samples, cut longwise to the muscle fibers, were fixed with Karnovskyâs solution (2.5% paraformaldehyde and 1.5% glutaraldehyde) in 0.1 M of dibasic phosphate (pH 7.2) at room temperature. Subsequently, the samples were rinsed twice with distilled water and dehydrated with increasing ethanol gradient solutions (e.g., 30%, 50%, 70%, 90%, and 100%) for 10 minutes in each solution at room temperature. The samples were then placed in 100% acetone for 10 min, dried with CO2 at the critical point condition (DCP-1 Critical Point Dryer; Denton Vacuum, Moorestown, NJ, USA), mounted in a holder with a two-sided adhesive tape, and coated with gold twice for 10 and 20 min. The ultrastructure was evaluated with the Phenom ProX scanning electron microscope (SEM; Thermofisher, Eindhovem, Netherlands) with an accelerating voltage of 5 kV and a working distance of 2 mm. SEM images were obtained at 1,000Ă magnification for fiber and 15,000Ă for sarcomere structure evaluation. Sarcomere length and muscle fiber diameter were calculated using the apparatus software.
Before Raman spectroscopic measurements, subcutaneous fat and perimysium were removed in all meat samples. Experiments were conducted with a LabRAM HR Evolution confocal microscope (Horiba Scientific, Villeneuve dâAscq, France) equipped with a 785 nm laser of 103 mW. All spectra were performed so that the incidence of the excitation laser beam was perpendicular to the direction of the muscle fibers. To improve the signal-to-noise ratio and to obtain the maximum exposure without saturation of the spectra, an integration time of 3 s and 40 accumulations were used, which is equivalent to a total measurement time of 15 min per loin. Raman spectra were preprocessed using SavitzkyâGolay smoothing filter with a polynomial of 3rd order and baseline corrected using rubber band baseline correction (RBC) in LabSpec 6 software. All measurements were performed in triplicates to obtain a representative sample.
The Studentâs t-test was used for independent samples to compare Tender and Tougher samples using animals as the experimental unit. Means of quality parameters were compared using Infostat software [28]. For all assays, the level of significance was set at 0.05.
To determine whether Raman spectra could predict meat and quality attributes, principal component analysis (PCA) was applied with the RamanToolSet free software [29]. The PCA model was calculated using a k-fold (k = 3) systematic cross-validation procedure to enhance model optimization. Pearson correlation coefficients were measured using the preprocessed Raman spectra as independent variables (âpredictorsâ or X variables) and the physicochemical values as dependent variables (Y variables).
Physicochemical parameters measured for meat quality characterization of Braford meat samples, such as hardness, cohesiveness, chewiness, WBSF, WHC, CL, IMF, fiber diameter, and sarcomere length, are listed in Table 1.
Hardness, WBSF, and cohesiveness values were higher in beef from tougher samples than that from tender ones (p< 0.0001, respectively), as was expected. Nevertheless, no differences in chewiness were observed between the groups. However, WHC and CL values were significantly different between groups, resulting in higher WHC and lower CL in the tougher meat samples compared to the tender ones with p<0.0001 and p = 0.0006, respectively. In addition, tender meat samples had a lower IMF content than the meat from the tougher group (p = 0.0174).
The data in Table 1 indicate that fiber diameter was similar between both groups (p = 0.1657). However, the Tougher group showed longer sarcomeres than the Tender samples (p<0.0001). The molecular architecture of the sarcomere as defined by the myosin filaments, the interaction with the actin filaments, and the boundaries formed by the Z-disks will subsequently influence basic meat quality traits such as tenderness and water-holding capacity [30].
In this regard, SEM images confirmed that the myofibrillar structure of the meat was very regular in both groups, with neighboring myofibrils adhering to each other and noticeable sarcomeres and Z-discs (Figure 1). Previously, was suggested that differences between tough and tender samples were associated with structural changes within myofibrils [9], and several authors described this difference using relationships between sarcomere length and tenderness [31,32]. In addition, hardness evaluated as WBSF, was higher in muscles with sarcomere lengths between 1.60 and 1.70 ÎŒm, while they were lower in muscles with sarcomere lengths of 1.40 to 1.50 ÎŒm, as previously reported for lamb meat [32].
The release of water from the meat could also be explained based on structural changes within the sarcomere, by the involvement of the degree of overlap of myosin and actin filaments and the number of cross-links between them during the development of rigor mortis, since with increasing sarcomere length, the degree of overlap of myosin and actin filaments becomes smaller and, therefore, so does the repulsion between filaments [30]. In this regard, as in a previous report [33], we found here that meat with longer sarcomeres has a higher WHC value. Ertbjerg and Puolanne [30] explained that the reduction in water retention at shorter sarcomere lengths is due to the stronger attraction created by the greater number of cross-bridges, and shorter sarcomeres produce longer distances between longitudinal filaments and thus less electrostatic repulsion.
Intramuscular fat has an indirect relationship to meat tenderness since data in Table 1 indicate that the tender group showed lower IMF content than the tougher samples. This results are in concordance with previous works made in Braford cattle bred [26,34,35]. This result could be associated with a prevalent lower protease activity due to the presence of IMF, which results in a decrease in meat tenderness [36].
Figure 2 shows the averaged Raman spectra of tender (black solid line) and tough (dashed black line) meat samples, with Raman signals typical of muscle tissue representing the major vibrations of amino acids chemical bonds, including tryptophan, tyrosine, and phenylalanine, as well as those protein backbone conformations and secondary structures [3]. The Raman spectrum of Tender and Tougher samples shows almost the same signal fingerprint, although slightly higher intensities were observed in the tougher than in the tender meat samples, in agreement with other studies [17,18].
Although it is not possible to assign with certainty any differential peak between the Raman spectra of beef and tender meat, a tentative interpretation of the spectra based on the evaluation of the âtough minus tender meatâ difference spectrum (gray top spectrum in Figure 2) is plausible. The Raman band centered near 1,653 cmâ1 represents the amide I band, which is an indicator of the overall concentration of proteins [11]. In turn, the vibrations centered near 1,250 cmâ1 represent the amide III band which is sensitive to secondary and tertiary structures of proteins [37]. The band at 1,446 cmâ1 is assigned to the âCH2 scissor, which decreases with increasing hydrophobicity in the molecular environment. The band at 1,003 cmâ1 is assigned to phenylalanine ring stretching, while bands centered around 900 and 1,130 cmâ1 are assigned to stretching modes of C-C and N from lipids and proteins, respectively [35]. Previously was demonstrated that the level of saturated fats increases with increasing levels of IMF [38], and this contributes to increase C-C stretching vibrations (1,020 to 1,130 cmâ1) and CH2 twists (1,300 cmâ1) [15], explaining differences observed in these regions in Raman spectra between toughest (with higher IMF) and tender meat.
Typical signals of meat which can be attributed to α-helical proteins are increased in tougher samples as indicated by peaks at 1,650, 1,447, 1,274, and 935 cmâ1. Furthermore, signals of myoglobin (1,540, 1,340, 1,126, and 885 cmâ1) and the aromatic amino acid side chains, notably tryptophan (1,557, 1,360, and 762 cmâ1) and tyrosine (1,610 and 855 cmâ1) are also enhanced in this group. Previously was reported that the tyrosine doublet signal weakened in tough meat is the direct reflection of less proteolysis [35]. Moreover, the tougher meat contains a higher proportion of ÎČ-sheet structure (1,665, 1,235, 1,006 cmâ1) than the tender meat [3]. The present results agree with these findings since tougher samples showed a higher α-helical to the ÎČ-sheet ratio in comparison to the tender meat. In this regard, when denaturation takes place the protein structures convert from α-helical into ÎČ-sheet or random coils, and thus, the Raman signal corresponding to α-helical becomes weaker [14].
The higher intensities observed in Raman spectrum of tougher samples can be understood by differences in packing of proteins as the Raman intensity is proportional to the number of scattering molecules; by an increase of the polarizability of the molecules due to changes in the surrounding or by a general change of the tissueâs optical properties leading to increased backscattering [17].
Raman fingerprinting can be used to construct chemometric models to classify and/or differentiate meat samples with distinct properties [39]. The first two principal components explained 83.28% of the variance between both groups of meat samples. The plot of the PCA scores shows that the tougher meat samples clustered in the positive space of PC1 and PC2, while the tender samples clustered in the negative space of PC1 and PC2 (Figure 3). The loadings plot in Figure 4 shows the Raman signatures responsible for the PCA discrimination. The Raman signals at 762, 855, 940, 1,003, 1,126, 1,360, 144, 1,540, 1,650 cmâ1 were associated with the highest variance. Though meat samples where PC1 and PC2 clustered in positive space were associated with phenylalanine (1,003 cmâ1), tryptophan (762 and 855 cmâ1), and myoglobin (1,126 and 1,540 cmâ1), which are stronger bands in these samples. Tender meat samples clustered in negative spaces PC1 and PC2 were associated with phenylalanine, α-helix, CH2-scissors (940 and 1,446 cmâ1), and amide I (1,650 cmâ1).
Figure 5 shows the correlation between meat quality traits obtained from Raman spectra and physicochemical measurements. The correlation coefficient is the unit of measurement used to calculate the intensity in the linear relationship between the variables involved. If the coefficient value lies between ±0.50 and ±1 is a strong correlation; if the value lies between ±0.30 and ±0.49 is a moderate correlation and the value lies below ±0.29, denotes a weak or no correlation. The analysis performed results in a strong correlation for hardness (R2 = 0.6983) and WBSF (R2 = 0.5832). And moderate or weak correlation for chewiness (R2 = 0.2650), cohesiveness (R2 = 0.0761), WHC (R2 = 0.0064), CL (R2 = 0.0089), and IMF (R2 = 0.0016).
Promising results were reported with Raman spectroscopy in lamb and pork meat. Schmidt et al [40] reported higher coefficients of determination (R2) of 0.72 and 0.86 when predicting shear force values from carcasses in longissimus thoracis and longissimus lumborum lamb muscles, respectively. Moreover, Beattie et al [37] reported R2 of 0.77 and 0.75 for predicting shear force in porcine longissimus and silverside bovine muscle, respectively. Nevertheless, Fowler et al [14] and Fowler et al [15] reported lower coefficient of determination with Raman spectra and shear force value in semimebranosus and longissimus lumborum (0.27 and 0.06, respectively) lamb muscle, and Santos et al [13] showed that sensory tenderness and shear force values in porcine muscle are weakly correlated (R2 = 0.2).
Our results gave a relatively good accuracy for predicting hardness (by TPA and WBSF) in comparison to previous Raman studies with different bovine muscles. For instance, Beattie et al [3] reported higher coefficients (R2 = 0.75) in silverside muscle, Chen et al [19] obtained R2 of 0.81 for tenderness in longissimus dorsi muscle from bulls, and Nian et al [41] found an R2 of 0.75 in predicting WBSF of young dairy bull beef. Nevertheless, Bauer et al [17] and Fowler et al [16] described lower R2 values (0.33 and 0.11) in the gluteus medius and longissimus lumborum muscles, respectively.
Nevertheless, our results showed a poor correlation with IMF (R2 = 0.0016). However, IMF was previously predicted with higher R2 in pork muscles and beef (R2 = 0.73 and 0.89, respectively) [18], suggesting that the amide I and III regions could predict this attribute due to the contribution from lipids including C-C stretching (1,660 to 1,640 cmâ1), âCH2 twisting (1,300 cmâ1), and CâH deformations (1,270 cmâ1).
Cama-Moncunill et al [18] also evaluated samples with different cooking loss values and reported that the Raman spectra recorded from samples with the lowest and highest cook-loss displayed little differences, which for the most part were observed at the region between 1,000 and 870 cmâ1 where samples with lowest cook-loss presented slightly higher intensity values. Compared to the present work, Cama-Moncunill et al [18] showed in a sample set performed with that aim that the cooking loss model exhibited high predictive ability (R2 = 0.67).
Furthermore, Pearson correlation coefficients between hardness and WBSF values and Raman intensity at each wavenumber of all spectra are shown in Figure 5. Pearson correlation coefficient at the signal pattern in the 1,250 to 1,650 cmâ1 was moderately correlated and at 1,003 cmâ1 was highly correlated to hardness (r = 0.84) and WBSF (r = 0.79), explaining this physical measured parameter. As was mentioned above, the signal pattern in the 1,250 to 1,600 cmâ1 includes some signals attributed to α-helical (1,650, 1,447, 1,274 cmâ1) and ÎČ-sheet structure (1,665, 1,235 cmâ1), associated with meat tenderness. Moreover, Beattie et al [37] found that the peak of the aromatic amino acids such as phenylalanine contributed to the correlation with shear force. The phenylalanine band at 1,003 cmâ1 is insensitive to the chemical and physical environment within the sample. This signal is sensitive to the relative abundance of this amino acid and so if the protein composition (or abundance) changes, the relative height of the phenylalanine band will change accordingly.
Although Raman spectroscopy is a robust and reproducible technique, it should be kept in mind that the prediction of meat quality by Raman spectroscopy is strongly influenced by several experimental issues, e.g., animal breed, muscle type, Raman device (handheld or benchtop), and acquisition characteristics (laser wavelength, integration time, accumulation of spectra, etc.), as well as the type of chemometric analysis selected, as can be seen in the literature. Therefore, Raman users should be aware of the above issues for the correct use of the technique in meat quality control.
To our knowledge, this is the first report that shows the capability of Raman spectroscopy in combination with PCA chemometric analysis to predict hardness (R2 = 0.698) and WBSF (R2 = 0.583) values in Braford longissimus thoracis et lumborum muscle. Furthermore, Raman spectroscopy is an adequate technique to identify Braford beef samples with differential tenderness attributes. Although the Raman pattern was similar in both tender and tougher meat samples, the signal intensity was higher in the latter, particularly for the peak due to phenylalanine at 1,003 cmâ1 and in the Amide III band, with a change in the ratio between the α-helical and ÎČ-sheet, suggesting that both the relative composition of the residues and the secondary structure of the meat proteins are affected. The high value of Pearsonâs correlation coefficient of hardness parameter with the Raman signal at 1,003 cmâ1, assigned to the symmetric vibration frequency of the phenylalanine ring, suggests that the content of this amino acid explains the differences between groups of samples. Results suggest that this technique could be a non-invasive and non-destructive method for the screening of individuals with tender meat. The meat industry would benefit from the ability to select animals that are superior and consistent in meat quality traits.
Notes
ACKNOWLEDGMENTS
The authors would like to acknowledge Mrs. Karina Moreno and PhD. Eduardo A. Parellada for the technical assistance in determining the physical parameters of beef.
Figure 1
Microestructure of myofibrilar structure in beef samples. Scaning electron microscopic (SEM) images were obtained using 1,000Ă magnification for tough (A) and tender (B) samples, while for sarcomere structure a magnification of 15,000Ă was selected for tough (C) and tender (D) samples.
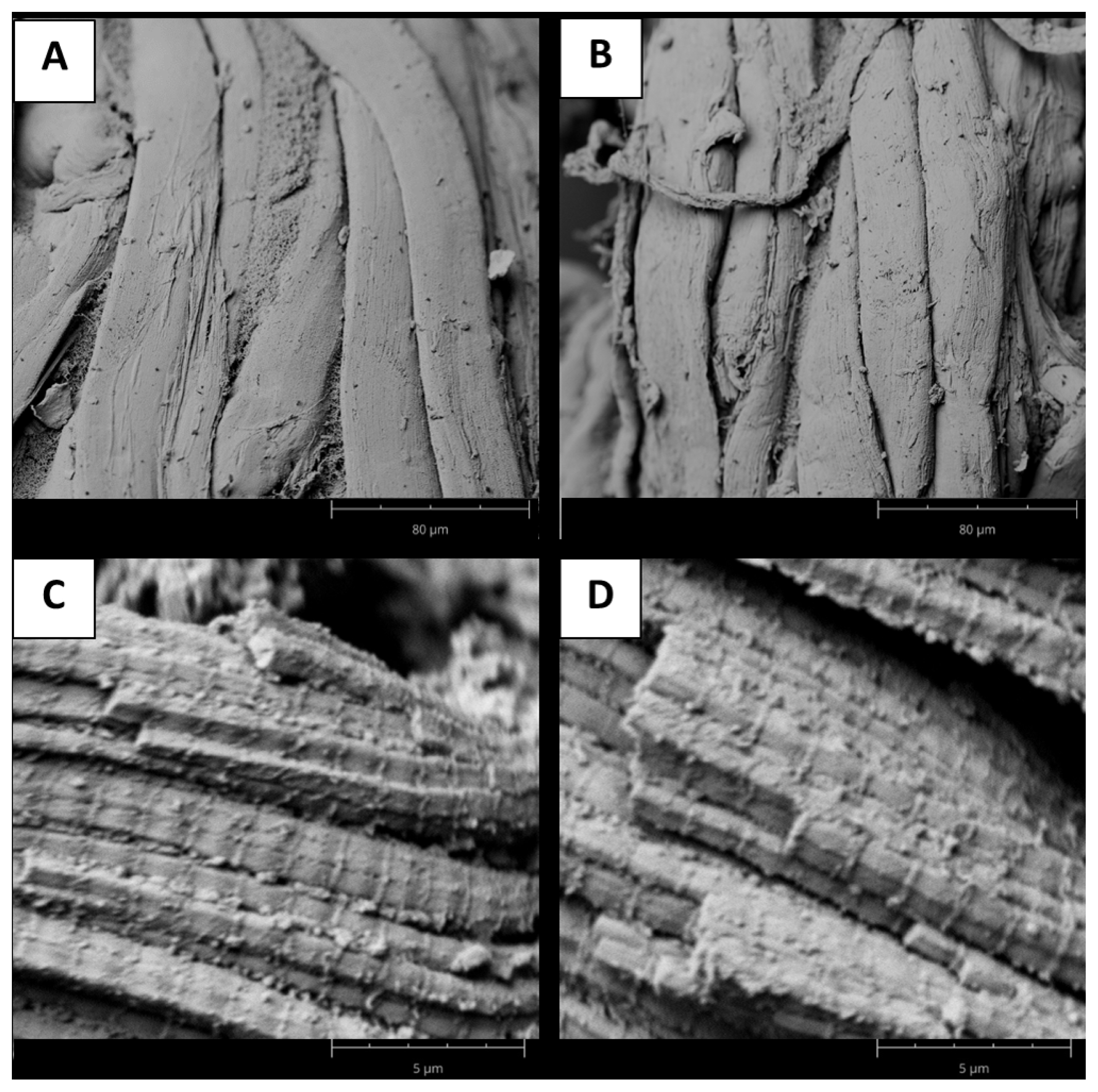
Figure 2
Averaged Raman spectra of the tenderest (black solid line) and the toughest (dashed black line) samples. The gray upper curve is the difference spectrum âtough minus tenderâ. Selected signals are indicated by symbols: ÎČ-shift proteins (star), α-helical proteins (diamond), myoglobin (circle), tryptophan, and tyrosine doublet (triangle).
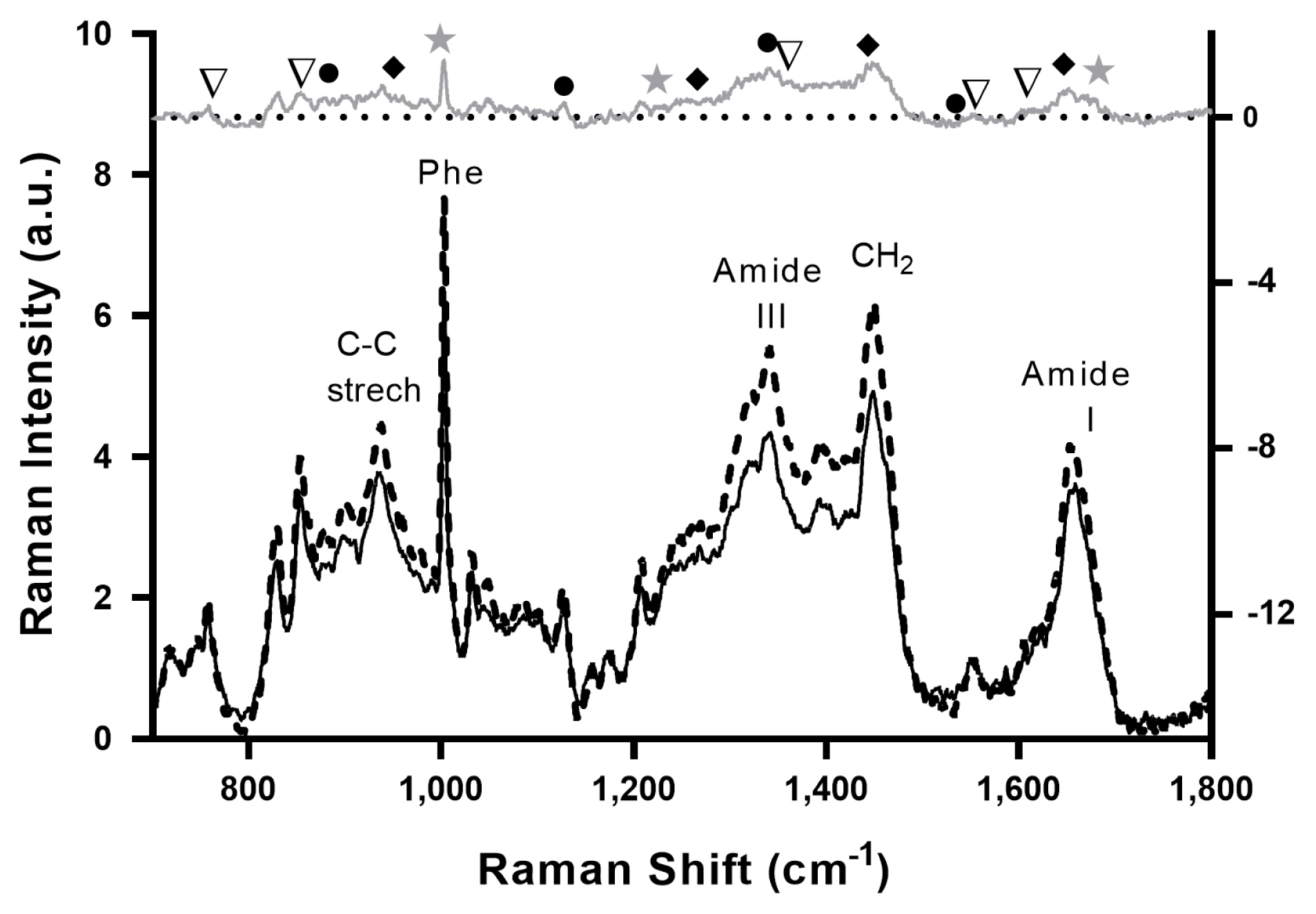
Figure 3
Principal component analysis (PCA) scores plot showing separation of tough (circle) and tender (square) meat samples in the principal component 1 (PC1) and 2 (PC2).
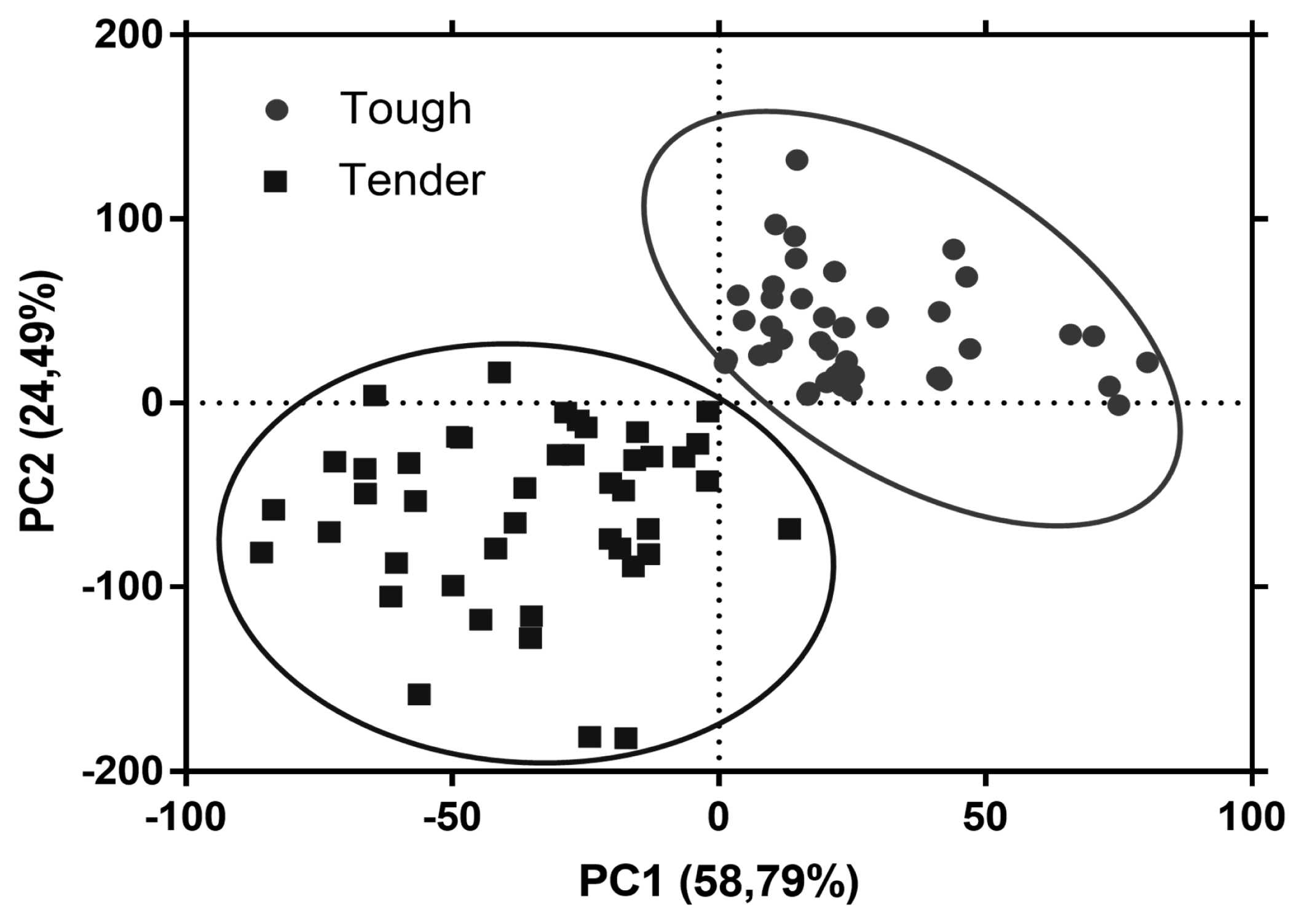
Figure 4
Principal component analysis (PCA) loadings plot with Raman spectral signatures associated with variation in tough and tender samples in component 1 (PC1) and 2 (PC2).

Figure 5
Relation between evaluated and predicted physical measurements using linear regression model. The relationship between evaluated and predicted physical measurements using linear regression model. R2 with a 95% confidence interval (dotted line), (A) Hardness, (B) Warner-Bratzler shear force (WBSF), (C) Chewiness, (D) Cohesiveness, (E) Water holding capacity (WHC), (F) Cooking loss (CL), (G) Intramuscular fat content (IMF), and (H) Pearson correlation coefficients (r) between Raman spectral data and hardness (gray) and WBSF (black) values; n = 30 samples.
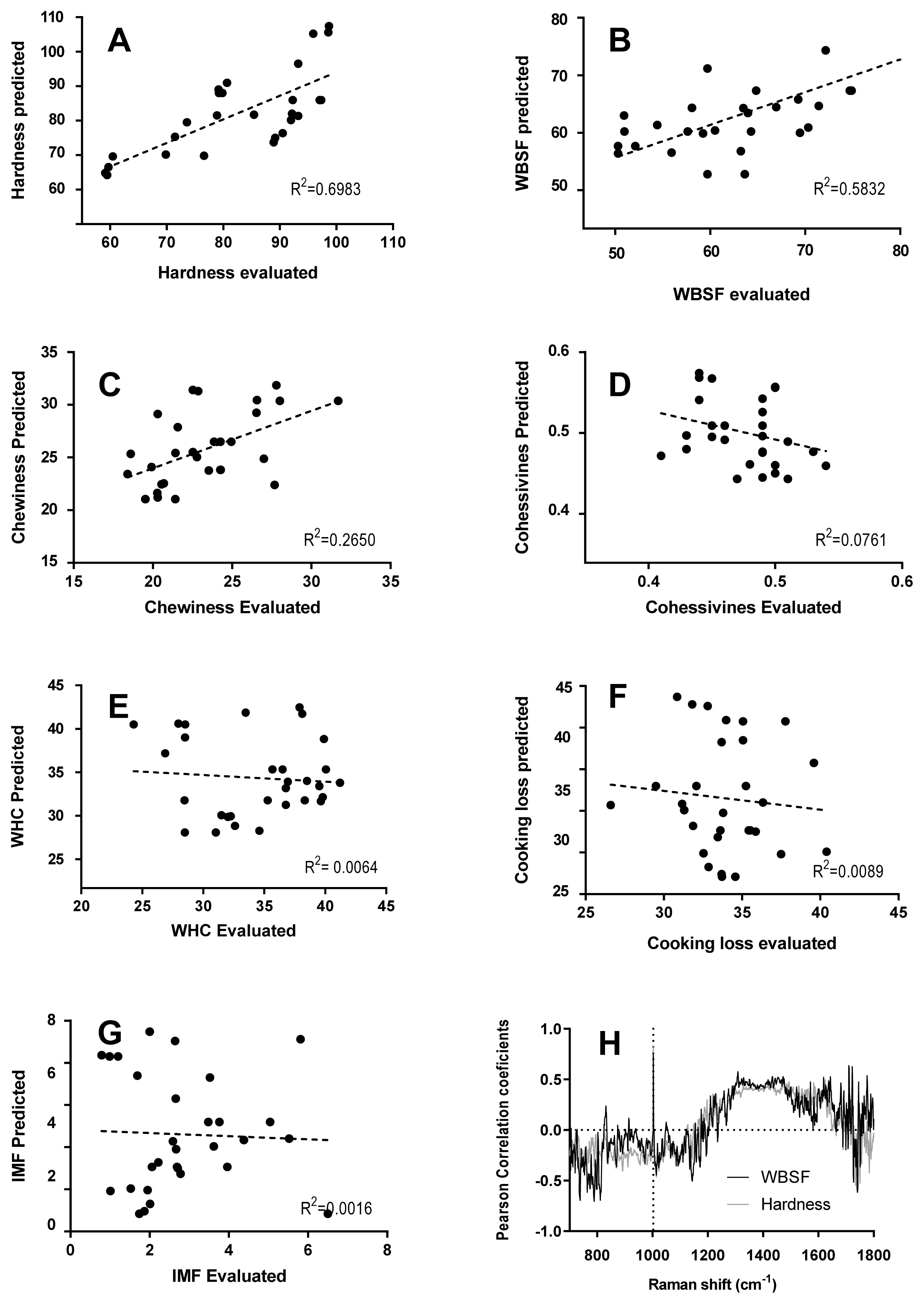
Table 1
Mean and standard deviation of the values of physical measurements of tender and tough meat samples
REFERENCES
1. Hunt MR, Garmyn AJ, OâQuinn TG, et al. Consumer assessment of beef palatability from four beef muscles from USDA Choice and Select graded carcasses. Meat Sci 2014; 98:1â8.
https://doi.org/10.1016/j.meatsci.2014.04.004


2. Miller MF, Carr Ma, Ramsey CB, Crockett KL, Hoover LC. Consumer thresholds for establishing the value of beef tenderness. J Anim Sci 2001; 79:3062â8.
https://doi.org/10.2527/2001.79123062x


3. Beattie RJ, Bell SJ, Farmer LJ, Moss BW, Patterson D. Preliminary investigation of the application of Raman spectroscopy to the prediction of the sensory quality of beef silverside. Meat Sci 2004; 66:903â13.
https://doi.org/10.1016/j.meatsci.2003.08.012


4. Ruiz De Huidobro F, Miguel E, BlĂĄzquez B, Onega E. A comparison between two methods (Warner-Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci 2005; 69:527â36.
https://doi.org/10.1016/j.meatsci.2004.09.008


5. Jeremiah LE, Dugan MER, Aalhus JL, Gibson LL. Assessment of the relationship between chemical components and palatability of major beef muscles and muscle groups. Meat Sci 2003; 65:1013â9.
https://doi.org/10.1016/S0309-1740(02)00309-1


6. Aalhus JL, Jeremiah LE, Dugan MER, Larsen IL, Gibson LL. Establishment of consumer thresholds for beef quality attributes. Can J Anim Sci 2004; 84:631â8.
https://doi.org/10.4141/A03-106

7. Ozbekova Z, Kulmyrzaev A. Fluorescence spectroscopy for accurate and rapid prediction of meat composition. Indian J Anim Sci 2019; 89:786â90.


8. Pu Y, Wang W, Alfano RR. Optical detection of meat spoilage using fluorescence spectroscopy with selective excitation wavelength. Appl Spectrosc 2013; 67:210â3.
https://doi.org/10.1366/12-06653


9. Damez JL, Clerjon S. Meat quality assessment using biophysical methods related to meat structure. Meat Sci 2008; 80:132â49.
https://doi.org/10.1016/j.meatsci.2008.05.039


10. De Biasio M, Stampfer P, Leitner R, Huck CW, Wiedemair V, Balthasar D. Micro-raman spectroscopy for meat type detection. In: Proceedings the Next-Generation Spectroscopic Technologies VIII. SPIE 2015; 9482:377â82.
https://doi.org/10.1117/12.2176321

11. Herrero AM. Raman spectroscopy a promising technique for quality assessment of meat and fish: a review. Food Chem 2008; 107:1642â51.
https://doi.org/10.1016/j.foodchem.2007.10.014

12. Wang Q, Lonergan SM, Yu C. Rapid determination of pork sensory quality using Raman spectroscopy. Meat Sci 2012; 91:232â9.
https://doi.org/10.1016/j.meatsci.2012.01.017


13. Santos CC, Zhao J, Dong X, et al. Predicting aged pork quality using a portable Raman device. Meat Sci 2018; 145:79â85.
https://doi.org/10.1016/j.meatsci.2018.05.021


14. Fowler SM, Schmidt H, van de Ven R, Wynn P, Hopkins DL. Raman spectroscopy compared against traditional predictors of shear force in lamb m. longissimus lumborum. Meat Sci 2014; 98:652â6.
https://doi.org/10.1016/j.meatsci.2014.06.042


15. Fowler SM, Ponnampalam EN, Schmidt H, Wynn P, Hopkins DL. Prediction of intramuscular fat content and major fatty acid groups of lamb M. longissimus lumborum using Raman spectroscopy. Meat Sci 2015; 110:70â5.
https://doi.org/10.1016/j.meatsci.2015.06.016


16. Fowler SM, Schmidt H, van de Ven R, Hopkins DL. Preliminary investigation of the use of Raman spectroscopy to predict meat and eating quality traits of beef loins. Meat Sci 2018; 138:53â8.
https://doi.org/10.1016/j.meatsci.2018.01.002


17. Bauer A, Scheier R, Eberle T, Schmidt H. Assessment of tenderness of aged bovine gluteus medius muscles using Raman spectroscopy. Meat Sci 2016; 115:27â33.
https://doi.org/10.1016/j.meatsci.2015.12.020


18. Cama-Moncunill R, Cafferky J, Augier C, et al. Prediction of Warner-Bratzler shear force, intramuscular fat, drip-loss and cook-loss in beef via Raman spectroscopy and chemometrics. Meat Sci 2020; 167:108157
https://doi.org/10.1016/j.meatsci.2020.108157


19. Chen Q, Zhang Y, Guo Y, et al. Non-destructive prediction of texture of frozen/thaw raw beef by Raman spectroscopy. J Food Eng 2020; 266:109693
https://doi.org/10.1016/j.jfoodeng.2019.109693

20. Fowler SM, Schmidt H, van de Ven R, Wynn P, Hopkins DL. Predicting meat quality traits of ovine m. semimembranosus, both fresh and following freezing and thawing, using a hand held Raman spectroscopic device. Meat Sci 2015; 108:138â44.
https://doi.org/10.1016/j.meatsci.2015.06.010


21. AOAC. Official methods of analysis. Association of Official Analytical Chemists. 15th edArlington, VA, USA: 1992.
22. Zamorano JM, Gambaruto M. Contribution to improving the meat water holding capacity test by the filter paper press method. A comparison of three methods for measuring areas. Meat Sci 1997; 46:129â37.
https://doi.org/10.1016/s0309-1740(97)00011-9


23. AMSA. Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat. Chicago, IL, USA: American Meat Science Asociation; 2015.
24. Ruiz de Huidobro F, Miguel E, Onega E, BlĂĄzquez B. Changes in meat quality characteristics of bovine meat during the first 6 days post mortem. Meat Sci 2003; 65:1439â46.
https://doi.org/10.1016/S0309-1740(03)00068-8


25. Szczesniak AS. Objective measurements of food texture. J Food Sci 1963; 28:410â20.
https://doi.org/10.1111/j.1365-2621.1963.tb00219.x

26. Coria MS, Reineri PS, Pighin D, et al. Feeding strategies alter gene expression of the calpain system and meat quality in the longissimus muscle of Braford steers. Asian-Australas J Anim Sci 2020; 33:753â62.
https://doi.org/10.5713/ajas.19.0163



27. Shackelford SD, Wheeler TL, Koohmaraie M. Tenderness classification of beef: I. Evaluation of beef longissimus shear force at 1 or 2 days postmortem as a predictor of aged beef tenderness. J Anim Sci 1997; 75:2417â22.
https://doi.org/10.2527/1997.7592417x


28. Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. InfoStat versiĂłn 2012 [internet]. Grupo InfoStat, FCA, Universidad Nacional de CĂłrdoba; Argentina: 2016. Available from: http://www.infostat.com.ar
29. Candeloro P, Grande E, Raimondo R, et al. Raman database of amino acids solutions: a critical study of extended multiplicative signal correction. Analyst 2013; 138:7331â40.
https://doi.org/10.1039/c3an01665j


30. Ertbjerg P, Puolanne E. Muscle structure, sarcomere length and influences on meat quality: A review. Meat Sci 2017; 132:139â52.
https://doi.org/10.1016/j.meatsci.2017.04.261


31. Hopkins DL, Toohey ES, Lamb TA, Kerr MJ, Van De Ven R, Refshauge G. Explaining the variation in the shear force of lamb meat using sarcomere length, the rate of rigor onset and pH. Meat Sci 2011; 88:794â6.
https://doi.org/10.1016/j.meatsci.2011.03.004


32. Pouliot E, GariĂ©py C, ThĂ©riault M, Castonguay FW. Use of electrical stimulation and chilling to enhance meat tenderness of heavy lambs. Can J Anim Sci 2014; 94:627â37.
https://doi.org/10.4141/cjas-2014-076

33. Hughes JM, Oiseth SK, Purslow PP, Warner RD. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci 2014; 98:520â32.
https://doi.org/10.1016/j.meatsci.2014.05.022


34. Realini CE, Duckett SK, Brito GW, Dalla Rizza M, De Mattos D. Effect of pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci 2004; 66:567â77.


35. Brito G, Lagomarsino X, Olivera J, et al. Effect of different feeding systems (pasture and supplementation) on carcass and meat quality of Hereford and Braford steers in Uruguay. In : Proc 54th ICOMST; 2008; p. 1â3.
36. Ueda Y, Watanabe A, Higuchi M, Shingu H, Kushibiki S, Shinoda M. Effects of intramuscular fat deposition on the beef traits of Japanese Black steers (Wagyu): Original article. Anim Sci J 2007; 78:189â94.
https://doi.org/10.1111/j.1740-0929.2007.00424.x

37. Beattie RJ, Bell SEJ, Borggaard C, Moss BW. Preliminary investigations on the effects of ageing and cooking on the Raman spectra of porcine longissimus dorsi. Meat Sci 2008; 80:1205â11.
https://doi.org/10.1016/j.meatsci.2008.05.016


38. Smith GC, Tatum JD, Belk KE. International perspective: Characterisation of United States Department of Agriculture and Meat Standards Australia systems for assessing beef quality. Aust J Exp Agric 2008; 48:1465â80.
https://doi.org/10.1071/EA08198

39. Velioglu HM, Temiz HT, Boyaci IH. Differentiation of fresh and frozen-thawed fish samples using Raman spectroscopy coupled with chemometric analysis. Food Chem 2015; 172:283â90.
https://doi.org/10.1016/j.foodchem.2014.09.073


40. Schmidt H, Scheier R, Hopkins DL. Preliminary investigation on the relationship of Raman spectra of sheep meat with shear force and cooking loss. Meat Sci 2013; 93:138â43.
https://doi.org/10.1016/j.meatsci.2012.08.019


41. Nian Y, Zhao M, OâDonnell CP, Downey G, Kerry JP, Allen P. Assessment of physico-chemical traits related to eating quality of young dairy bull beef at different ageing times using Raman spectroscopy and chemometrics. Food Res Int 2017; 99:778â89.
https://doi.org/10.1016/j.foodres.2017.06.056








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





