 |
 |
| Anim Biosci > Volume 36(3); 2023 > Article |
|
Abstract
Objective
Methods
Results
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This research was financially supported by IPET (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries) through the Advanced Production Technology Development Program (grant No: 118016-03, Ministry of Agriculture, Food and Rural Affairs), and by a Korea Research Fellowship (KRF) Program (grant No: 2019H1D3A1A01102738) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
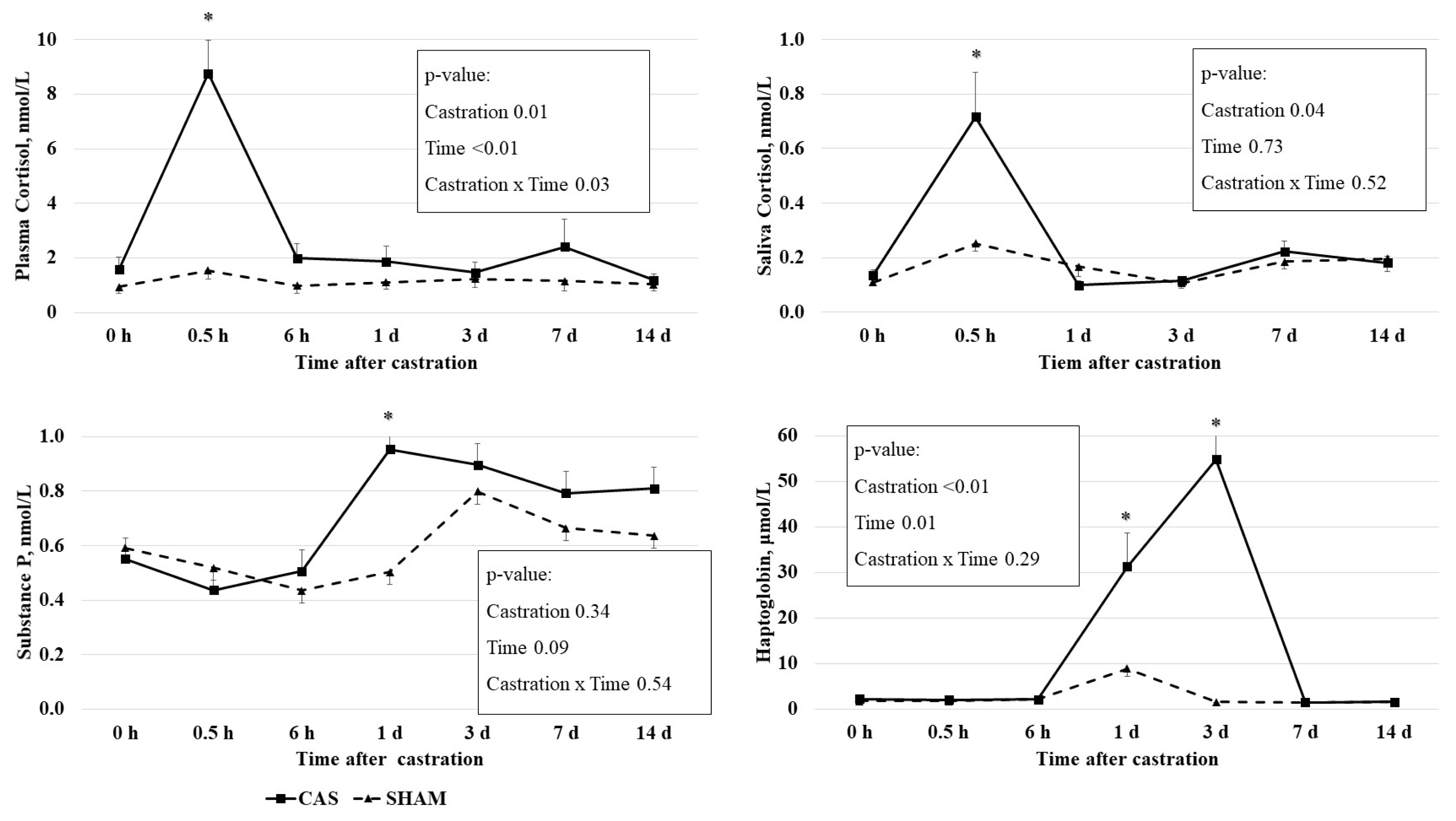
Figure┬Ā1
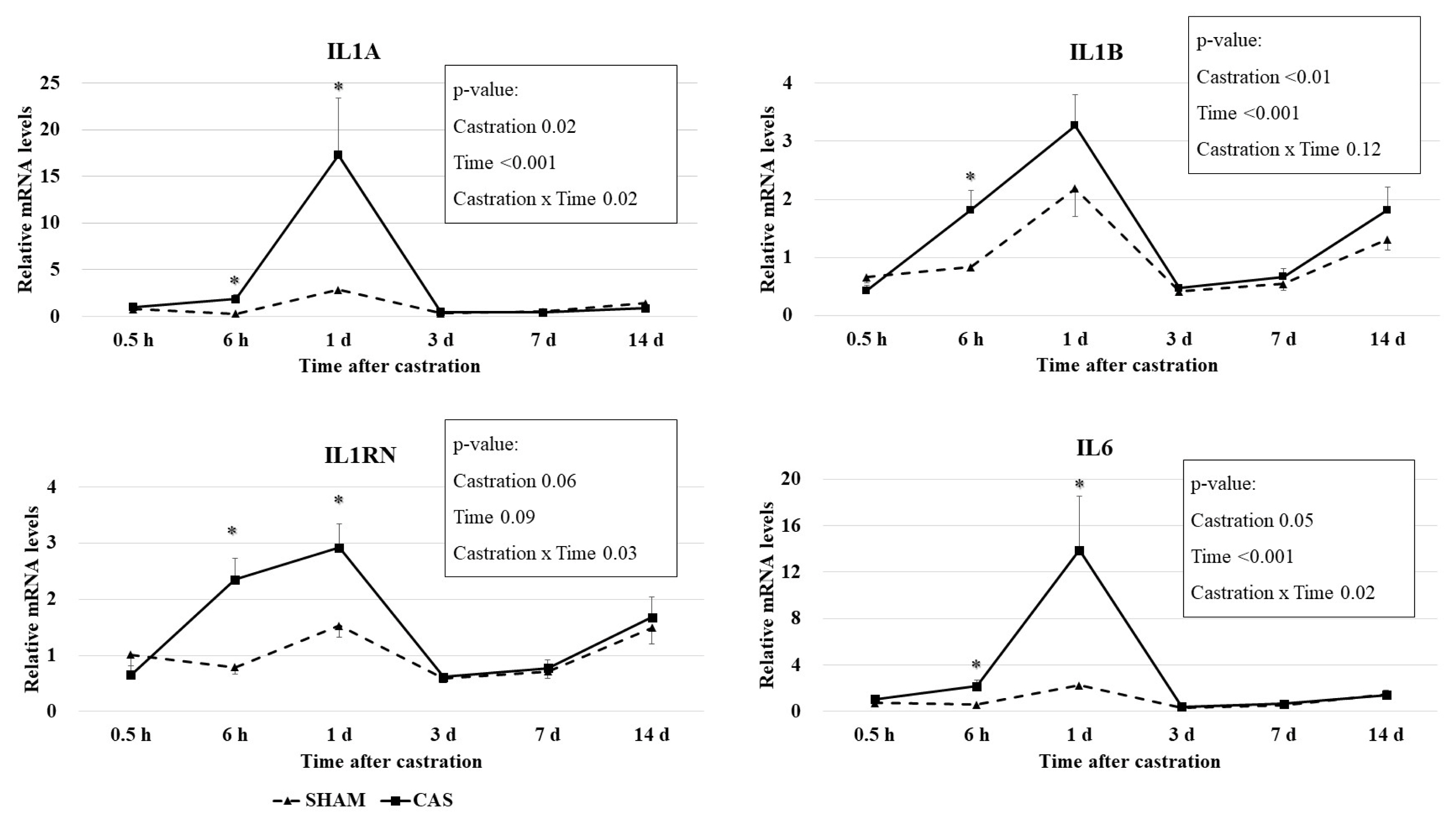
Figure┬Ā2
Table┬Ā1
| Ingredient | Percentage | Chemical composition | Percentage |
|---|---|---|---|
|
|
|
||
| Concentrate ingredients (DM basis) | DM | 92.0 | |
| Ground corn | 15.82 | Crude ash | 7.145 |
| Ground wheat | 18.00 | CP | 15.57 |
| Salt | 0.88 | EE | 4.08 |
| Molasses | 5.50 | ADF | 11.49 |
| Wheat bran | 3.00 | NDF | 27.495 |
| Corn flour | 5.00 | NFC2) | 45.71 |
| Rice bran | 3.00 | Calcium | 1.21 |
| Ammonium chloride | 0.15 | Phosphate | 0.47 |
| Condensed molasses solubles | 1.50 | TDN3) (%) | 88.705 |
| Cottonseed hulls | 1.50 | DE4) (Mcal/kg) | 3.91 |
| Porphyry | 2.00 | ME5) (Mcal/kg) | 3.46 |
| Rapeseed meal | 2.22 | Timothy composition | |
| Limestone | 3.30 | ŌĆāDM | 95.54 |
| Corn gluten feed | 8.50 | ŌĆāCrude ash | 6.77 |
| Dried distilled grain solubles | 9.38 | ŌĆāCP | 11.01 |
| Palm kernel meal | 10.00 | ŌĆāEE | 1.42 |
| Copra meal | 10.00 | ŌĆāCalcium | 0.28 |
| Mineral/vitamin premix1) | 0.25 | ŌĆāPhosphate | 0.275 |
| Total | 100.00 | ŌĆāADF | 35.68 |
| ŌĆāNDF | 63.05 | ||
DM, dry matter; CP, crude protein; EE, ether extract; ADF, acid detergent fiber; NDF, neutral detergent fiber; NFC, non fiber carbohydrate; TDN, total digestible nutrient; DE, digestible energy; ME, metabolizable energy.
Table┬Ā2
Table┬Ā3
| Item (sample number)2) | Plasma and saliva items | mRNA levels | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| PC (133) | SC (114) | SP (133) | HP (133) | IL1A (114) | IL1B (114) | IL1RN (114) | IL6 (114) | |
| PC (133) | - | |||||||
| SC (114) | 0.69** | - | ||||||
| SP (133) | ŌłÆ0.06 | ŌłÆ0.18 | - | |||||
| HP (133) | ŌłÆ0.06 | ŌłÆ0.11 | 0.25** | - | ||||
| IL1A (114) | ŌłÆ0.03 | ŌłÆ0.10 | 0.17 | ŌłÆ0.10 | - | |||
| IL1B (114) | ŌłÆ0.12 | ŌłÆ0.15 | 0.04 | ŌłÆ0.07 | 0.55** | - | ||
| IL1RN (114) | ŌłÆ0.06 | ŌłÆ0.08 | 0.14 | ŌłÆ0.10 | 0.48** | 0.83** | - | |
| IL6 (114) | ŌłÆ0.02 | ŌłÆ0.10 | 0.14 | ŌłÆ0.13 | 0.96** | 0.55** | 0.67** | |
PC, plasma cortisol; SC, saliva cortisol; SP, plasma substance P; HP, plasma haptoglobin; IL1A, interleukin-1 alpha; IL1B, interleukin-1 beta; IL1RN, interleukin-1 receptor antagonist; IL6, interleukin-6.
2) Numerical values in parenthesis indicate the number of samples used as follows. A total 133 samples of each of PC, SP, and HP were used in the correlation analysis [19 animals├Ś7 time points (0 h, 0.5 h, 6 h, 1 d, 3 d, 7 d, and 14 d)]. A total of 114 samples of SC were used in the correlation analysis [19 animals ├Ś 6 time points (0 h, 0.5 h, 1 d, 3 d, 7 d, and 14 d)]. A total of 114 samples of each of mRNA level for the four genes were used in the correlation analysis [19 animals ├Ś 6 time points (0.5 h, 6 h, 1 d, 3 d, 7 d, and 14 d)]. The number of actual correlation pairs were 133, 114, or 95, depending on the two parameters being compared.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





