 |
 |
| Anim Biosci > Volume 36(5); 2023 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Notes
SUPPLEMENTARY MATERIAL
Figure 1
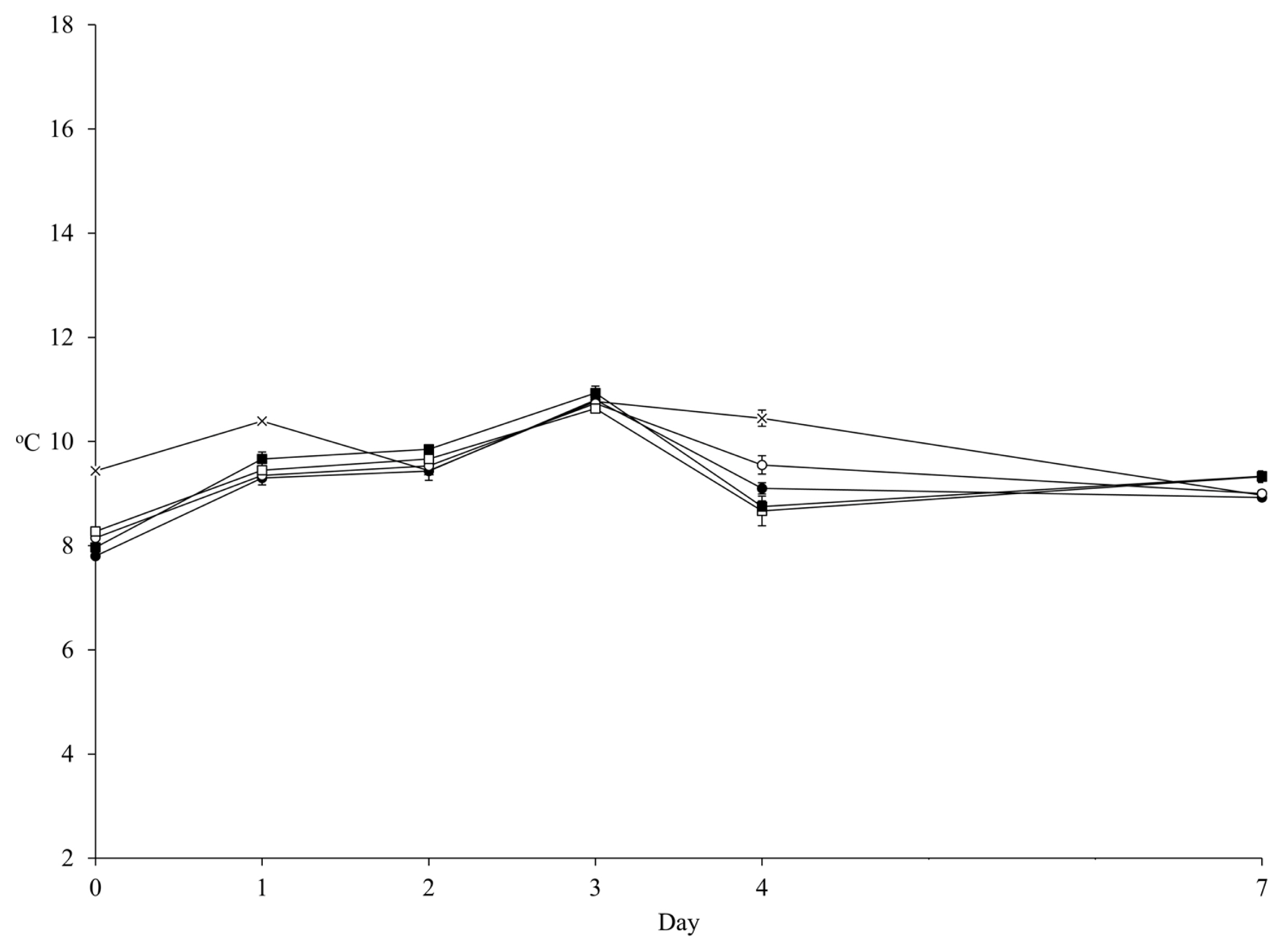
Figure 2
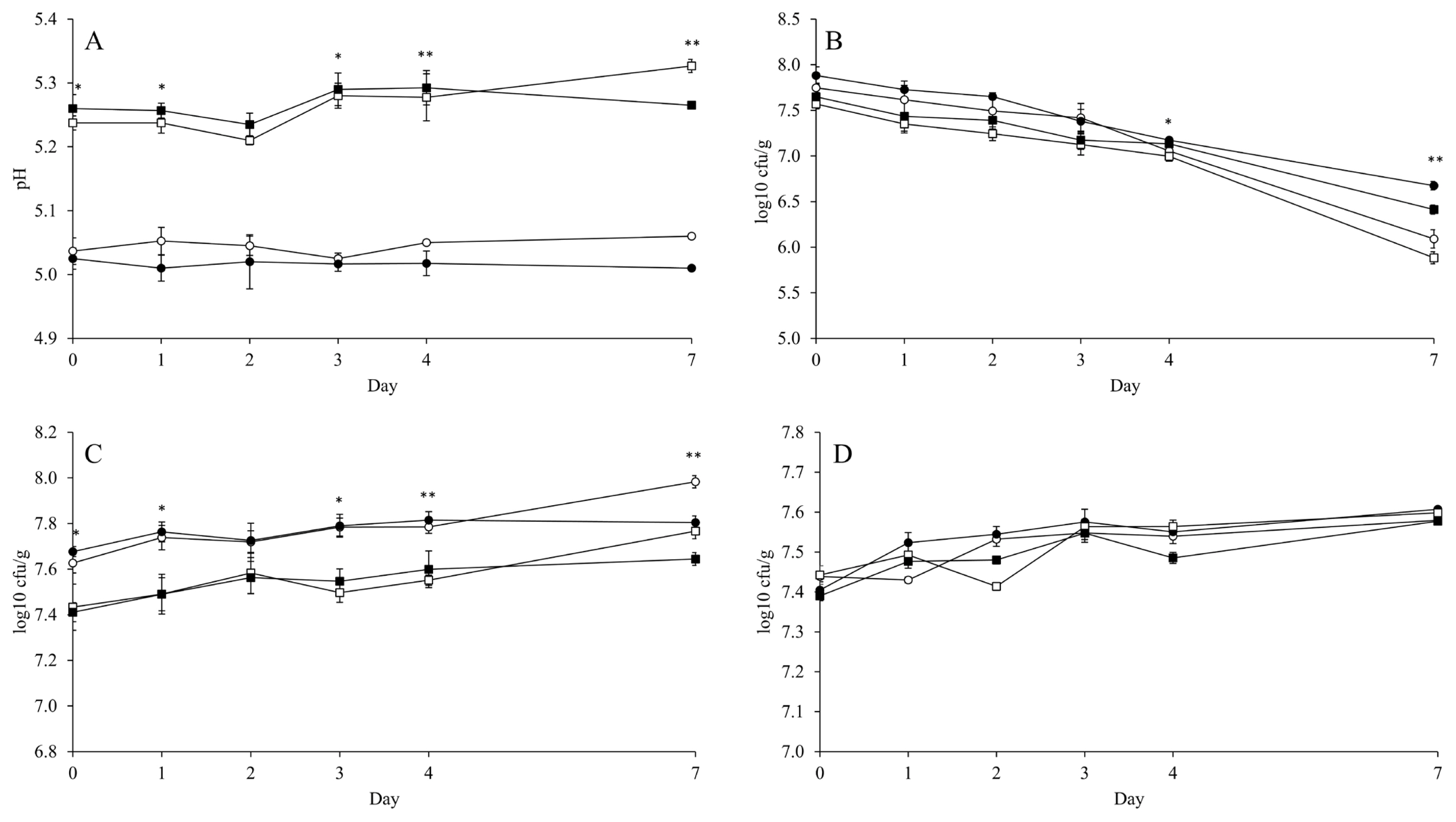
Table 1
| Item | LOW1) | HIGH | ||
|---|---|---|---|---|
|
|
|
|||
| CON | MIX | CON | MIX | |
| Corn silage | 14.5 | 14.5 | 11.1 | 11.1 |
| Sudangrass silage | 12.1 | 12.1 | 9.20 | 9.20 |
| Rice straw | 25.2 | 25.2 | 19.2 | 19.2 |
| Corn meal | 31.7 | 31.7 | 26.4 | 26.4 |
| Corn gluten feed | 8.49 | 8.49 | 11.7 | 11.7 |
| Soybean meal | 4.64 | 4.64 | 5.15 | 5.15 |
| Lupin seed | 2.41 | 2.41 | 5.87 | 5.87 |
| Palm kernel | 0.00 | 0.00 | 7.58 | 7.58 |
| DDGS | 0.00 | 0.00 | 1.90 | 1.90 |
| Molasses | 0.48 | 0.48 | 0.72 | 0.72 |
| Premix2) | 0.50 | 0.50 | 1.19 | 1.19 |
1) LOW, low energy diet; HIGH, high energy diet; CON, fermented total mixed ration with corn silage applied no inoculant; MIX, fermented total mixed ration with corn silage applied inoculant mixture of L. brevis 5M2 and L. buchneri 6M1.
2) One kilogram contained the following: vitamin A, 450,000 IU; vitamin D3, 45,000 IU; vitamin E, 1,500 IU; pantothenic acid, 40 mg; niacin, 30 mg; biotin, 20 mg; folic acid, 10 mg; FeSO4, 3,600 mg; CoSO4, 150 mg; CuSO4, 4,500 mg; MnSO4, 1,500 mg; ZnSO4, 2,200 mg; I, 400 mg; Se (Na), 150 mg; limestone, 2,000 mg; salt, 650 mg.
Table 2
| Item | LOW1) | HIGH | SEM | Contrast2) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CON | MIX | CON | MIX | INO | DIET | INO×DIET | ||
| Dry matter | 65.3b | 65.3b | 68.5a | 68.4a | 0.142 | 0.768 | <0.001 | 0.860 |
| Crude protein | 12.7b | 12.6b | 13.4a | 13.5a | 0.160 | 0.857 | <0.001 | 0.226 |
| Ether extract | 3.27a | 3.35a | 2.70b | 2.86b | 0.332 | 0.506 | 0.009 | 0.838 |
| Crude ash | 12.0a | 12.0a | 11.0b | 10.5b | 0.353 | 0.279 | <0.001 | 0.279 |
| Neutral detergent fiber | 42.3a | 42.4a | 32.4b | 32.5b | 0.294 | 0.658 | <0.001 | 0.706 |
| Acid detergent fiber | 22.3a | 22.0a | 14.9b | 14.9b | 0.381 | 0.540 | <0.001 | 0.578 |
| Hemicellulose | 20.0a | 20.3a | 17.5b | 17.6b | 0.446 | 0.893 | <0.001 | 0.933 |
| IVDMD | 70.5b | 70.4b | 74.0a | 73.9a | 0.962 | 0.907 | <0.001 | 0.954 |
| IVNDFD | 50.3b | 49.7b | 52.7a | 52.3a | 0.543 | 0.242 | 0.001 | 0.931 |
| TDN | 71.3b | 71.2b | 77.1a | 77.0a | 0.114 | 0.946 | <0.001 | 0.975 |
SEM, standard error of mean; DM, dry matter; IVDMD, in vitro dry matter digestibility; IVNDFD, in vitro neutral detergent fiber digestibility; TDN, total digestible nutrients.
1) LOW, low energy diet; HIGH, high energy diet; CON, fermented total mixed ration with corn silage applied no inoculant; MIX, fermented total mixed ration with corn silage applied inoculant mixture of L. brevis 5M2 and L. buchneri 6M1.
Table 3
| Item | LOW1) | HIGH | SEM | Contrast2) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CON | MIX | CON | MIX | INO | DIET | INO×DIET | ||
| Dry matter | 64.0b | 64.2b | 67.5a | 67.4a | 0.488 | 0.946 | <0.001 | 0.900 |
| Crude protein | 10.9b | 11.0b | 12.7a | 13.0a | 0.260 | 0.071 | <0.001 | 0.444 |
| Ether extract | 3.40a | 3.37a | 2.78b | 2.73b | 0.144 | 0.646 | <0.001 | 0.892 |
| Crude ash | 11.5a | 11.6a | 10.1b | 9.91b | 0.572 | 0.644 | <0.001 | 0.841 |
| Neutral detergent fiber | 44.8a | 45.0a | 33.5b | 33.0b | 2.535 | 0.908 | <0.001 | 0.787 |
| Acid detergent fiber | 23.7a | 21.7a | 16.0b | 15.2b | 0.928 | 0.032 | <0.001 | 0.291 |
| Hemicellulose | 21.1a | 23.3a | 17.5b | 17.7b | 0.954 | 0.104 | <0.001 | 0.216 |
| IVDMD | 59.9d | 62.0c | 65.9b | 69.0a | 0.501 | <0.001 | <0.001 | 0.122 |
| IVNDFD | 49.7d | 52.2c | 55.2b | 58.5a | 0.789 | 0.001 | <0.001 | 0.415 |
DM, dry matter; SEM, standard error of mean; IVDMD, in vitro dry matter digestibility; IVNDFD, in vitro neutral detergent fiber digestibility.
1) LOW, low energy diet; HIGH, high energy diet; CON, fermented total mixed ration with corn silage applied no inoculant; MIX, fermented total mixed ration with corn silage applied inoculant mixture of L. brevis 5M2 and L. buchneri 6M1.
Table 4
| Item | LOW1) | HIGH | SEM | Contrast2) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CON | MIX | CON | MIX | INO | DIET | INO×DIET | ||
| Fermentation indices | ||||||||
| pH | 5.04b | 5.03b | 5.24a | 5.26a | 0.035 | 0.779 | <0.001 | 0.383 |
| Ammonia-N (% DM) | 0.04b | 0.04b | 0.07a | 0.06a | 0.003 | 0.524 | <0.001 | 1.000 |
| Lactate (% DM) | 1.57ab | 1.49b | 1.78a | 1.66ab | 0.100 | 0.157 | 0.013 | 0.773 |
| Acetate (% DM) | 2.13b | 2.38b | 3.79a | 4.01a | 0.072 | 0.010 | <0.001 | 0.748 |
| Propionate (% DM) | ND | ND | ND | ND | NA | NA | NA | NA |
| Butyrate (% DM) | ND | ND | ND | ND | NA | NA | NA | NA |
| Lactate:acetate | 0.74a | 0.63a | 0.47b | 0.41b | 0.045 | 0.078 | <0.001 | 0.142 |
| Microbial counts (log10 cfu/g) | ||||||||
| Lactic acid bacteria | 7.75a | 7.88a | 7.57b | 7.65b | 0.156 | 0.240 | 0.036 | 0.757 |
| Bacillus | 7.44 | 7.40 | 7.44 | 7.39 | 0.111 | 0.471 | 0.966 | 0.883 |
| Yeast | 7.63a | 7.68a | 7.43b | 7.41b | 0.123 | 0.871 | 0.005 | 0.587 |
| Mold | ND | ND | ND | ND | NA | NA | NA | NA |
SEM, standard error of mean; DM, dry matter; ND, not detected; cfu, colony forming unit; NA, not applicable.
1) LOW, low energy diet; HIGH, high energy diet; CON, fermented total mixed ration with corn silage applied no inoculant; MIX, fermented total mixed ration with corn silage applied inoculant mixture of L. brevis 5M2 and L. buchneri 6M1.
Table 5
| Item | LOW1) | HIGH | SEM | Contrast2) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CON | MIX | CON | MIX | INO | DIET | INO×DIET | ||
| pH | 5.04b | 5.02b | 5.26a | 5.27a | 0.025 | <0.001 | 0.556 | 0.066 |
| Microbial counts (log10 cfu/g) | ||||||||
| Lactic acid bacteria | 7.24ab | 7.41a | 7.03b | 7.20b | 0.106 | 0.001 | 0.013 | 0.979 |
| Bacillus | 7.51 | 7.53 | 7.51 | 7.49 | 0.069 | 0.598 | 0.972 | 0.647 |
| Yeast | 7.77a | 7.76a | 7.55b | 7.54b | 0.026 | <0.001 | 0.342 | 0.451 |
1) LOW, low energy diet; HIGH, high energy diet; CON, fermented total mixed ration with corn silage applied no inoculant; MIX, fermented total mixed ration with corn silage applied inoculant mixture of L. brevis 5M2 and L. buchneri 6M1.
Table 6
| Item1) | LOW2) | HIGH | SEM | Contrast3) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CON | MIX | CON | MIX | INO | DIET | INO×DIET | ||
| A (mL/g DM) | 1.77b | 1.95ab | 2.00a | 1.93ab | 0.089 | 0.699 | 0.041 | 0.036 |
| B (mL/g DM) | 2.36b | 2.47b | 3.12a | 3.49a | 0.166 | 0.018 | <0.001 | 0.221 |
| A+B (mL/g DM) | 4.16b | 4.41b | 5.12a | 5.42a | 0.143 | 0.009 | <0.001 | 0.786 |
| C (%/h) | 0.22 | 0.21 | 0.21 | 0.19 | 0.017 | 0.141 | 0.204 | 0.558 |
| L (h) | 4.66a | 4.74a | 4.23b | 3.76b | 0.239 | 0.070 | <0.001 | 0.095 |
1) A, the immediately fermentable fraction; B, the potentially fermentable fraction; A+B, the total fermentable fraction; C, the fractional fermentation rate; L, the lag phase.
2) LOW, low energy diet; HIGH, high energy diet; CON, fermented total mixed ration with corn silage applied no inoculant; MIX, fermented total mixed ration with corn silage applied inoculant mixture of L. brevis 5M2 and L. buchneri 6M1.
Table 7
| Item | LOW1) | HIGH | SEM | Contrast2) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CON | MIX | CON | MIX | INO | DIET | INO×DIET | ||
| pH | 6.88a | 6.84ab | 6.75bc | 6.68c | 0.042 | 0.034 | <0.001 | 0.397 |
| Ammonia-N (mg/dL) | 24.7ab | 23.6b | 26.4a | 25.4ab | 1.202 | 0.091 | 0.004 | 0.793 |
| Total VFA (mM/L) | 107.9b | 114.4b | 119.4ab | 129.2a | 5.042 | 0.014 | 0.001 | 0.584 |
| Acetate, % molar | 67.4 | 67.5 | 66.5 | 66.5 | 0.755 | 0.875 | 0.033 | 0.892 |
| Propionate | 16.9 | 16.7 | 16.8 | 17.0 | 0.858 | 0.990 | 0.839 | 0.713 |
| Iso-butyrate | 0.94 | 1.09 | 0.98 | 0.93 | 0.115 | 0.452 | 0.250 | 0.116 |
| Butyrate | 11.5 | 11.8 | 12.9 | 12.9 | 0.671 | 0.681 | 0.005 | 0.695 |
| Iso-valerate | 3.27a | 2.88ab | 2.81ab | 2.67b | 0.210 | 0.040 | 0.018 | 0.265 |
| Acetate:propionate | 3.99 | 4.03 | 3.95 | 3.93 | 0.299 | 0.950 | 0.551 | 0.772 |
1) LOW, low energy diet; HIGH, high energy diet; CON, fermented total mixed ration with corn silage applied no inoculant; MIX, fermented total mixed ration with corn silage applied inoculant mixture of L. brevis 5M2 and L. buchneri 6M1.
REFERENCES
- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print





