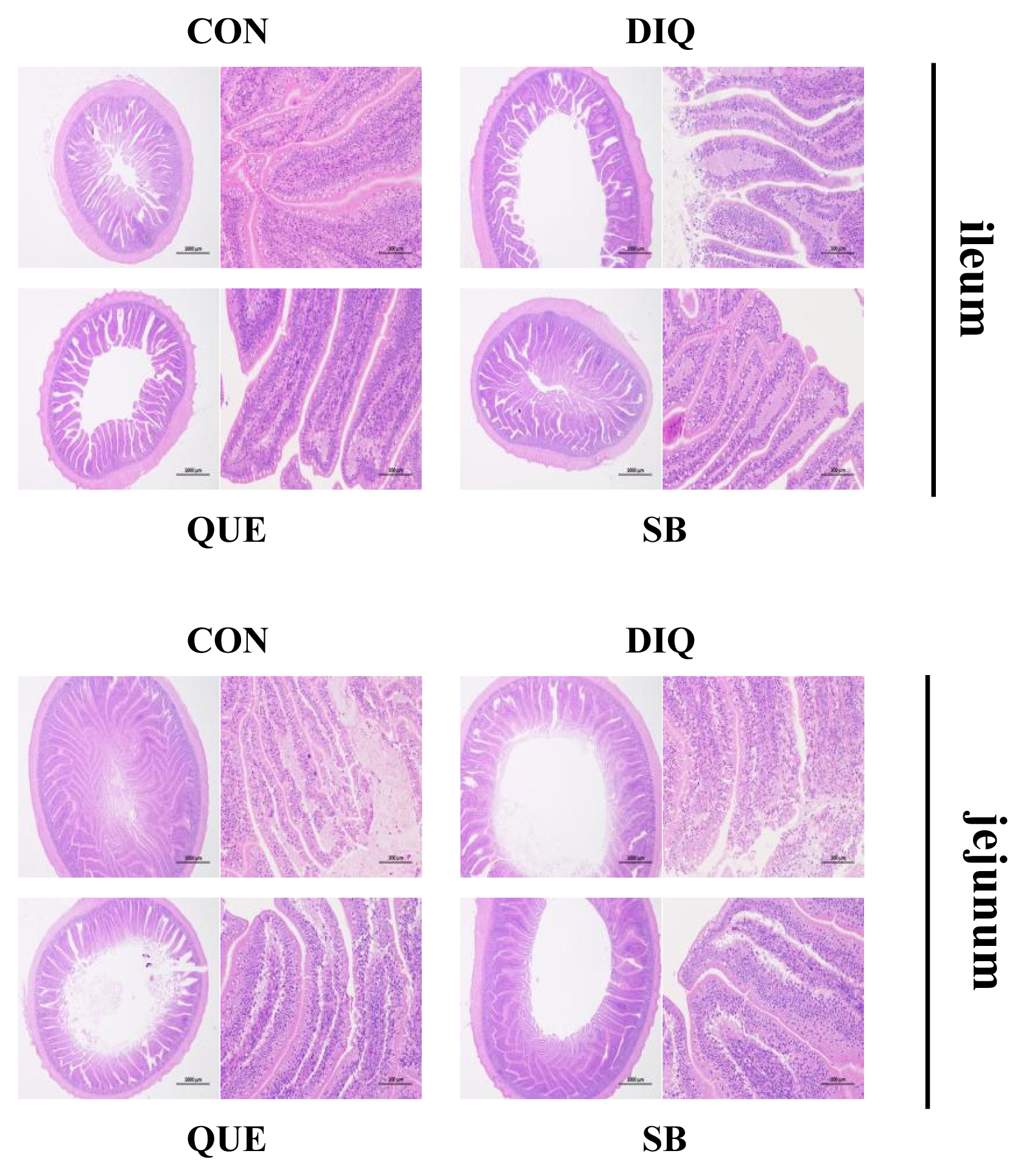
Diquat-induced oxidative stress has been reported to affect intestinal morphology and antioxidant capacity in animals and play a principal role in disrupting intestinal function and breaking oxidative balance [
9,
10,
20]. In this investigation, we observed that serum MDA concentration of samples was increased in DIQ group on the first and tenth day. The other main anti-oxidative parameters, SOD, GSH-PX, and T-AOC, in the CON group were lower in the DIQ group on the first and tenth days, which was consistent with previous reports [
8ŌĆō
10]. Quercetin is one of the most widely distributed bioflavonoids and exhibits metal chelation and free-radical scavenging activities to fight oxidative stress [
21]. A previous study demonstrated that supplementation with QUE could relieve the upregulation of MDA and downregulation of GSH-PX and SOD in jejunal tissue [
14]. Quercetin treatment was shown to be beneficial for decreasing MDA levels in transported pigs [
22]. These previous results are similar to the observations made on the tenth day of the present study. Butyric acid has been shown to play an important role in maintaining the integrity of the intestinal mucosa and exerting potent antioxidative effects in animals [
19]. A study found that serum GSH-PX activity of calves linearly increased and serum MDA concentration linearly decreased after sodium butyrate supplementation during the entire experimental period [
23], which is similar to our results. In addition, serum MDA levels were significantly lower after sodium butyrate supplementation on the first day, which was noted previously in stressed broiler chickens supplemented with microencapsulated NaB [
18].
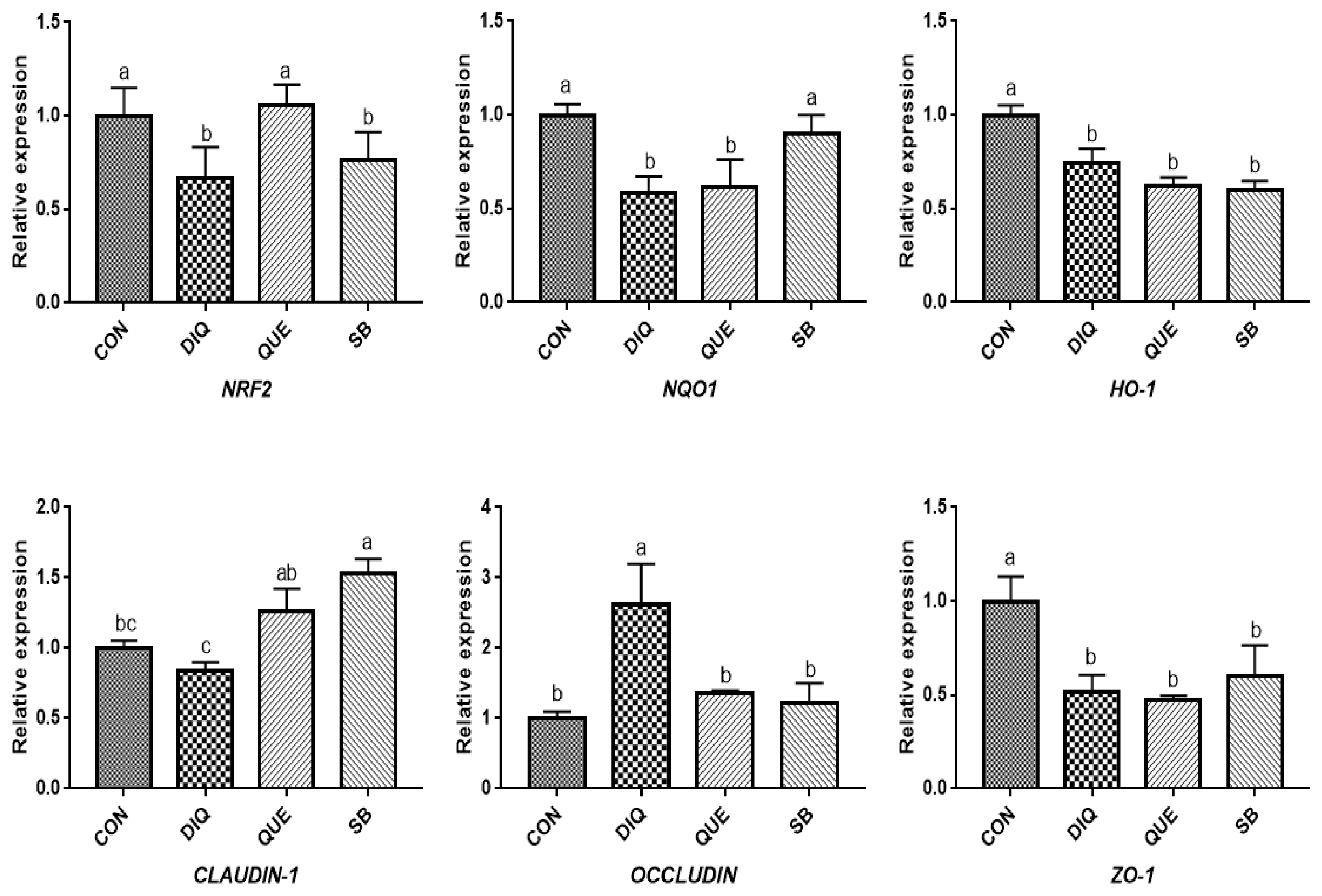
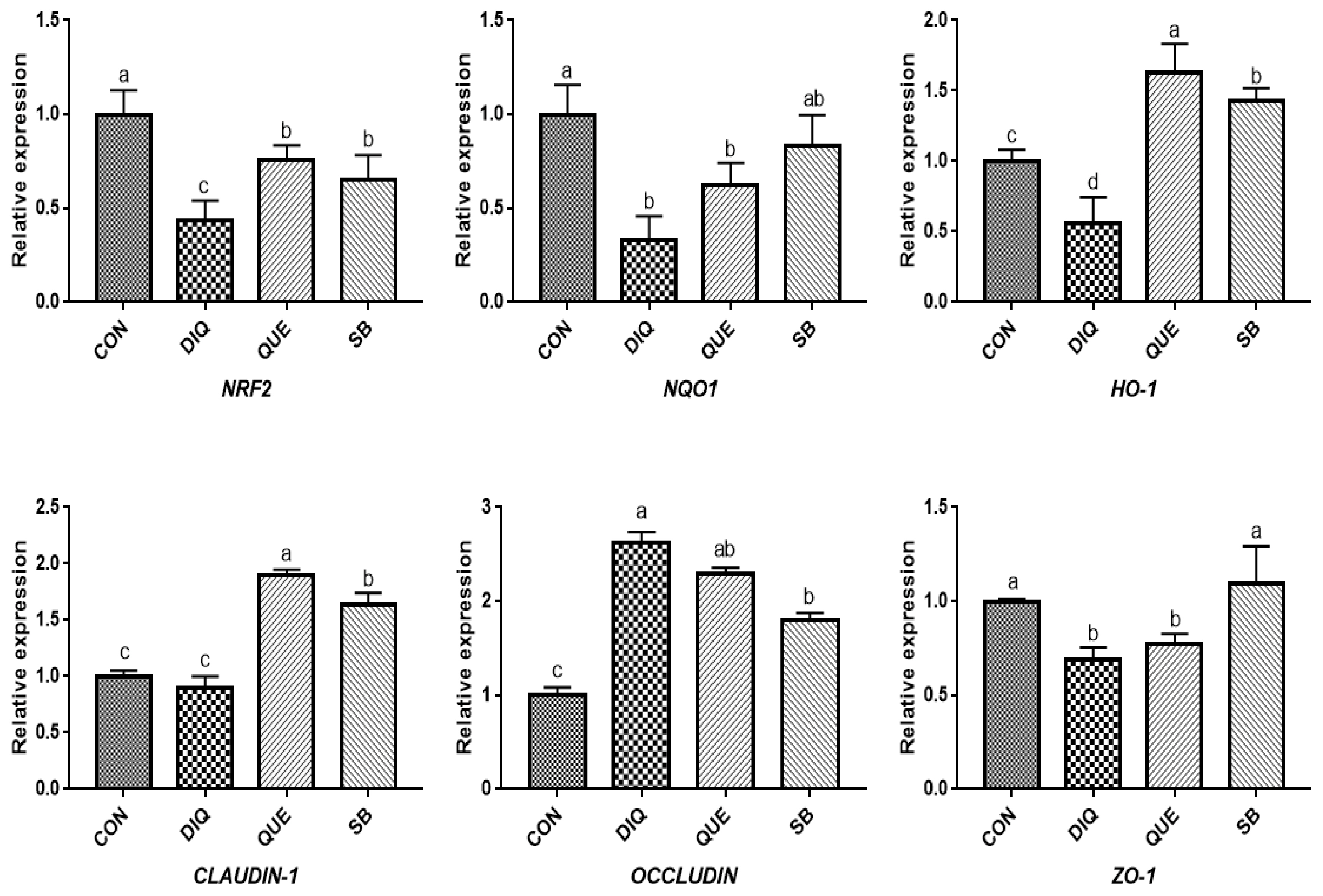
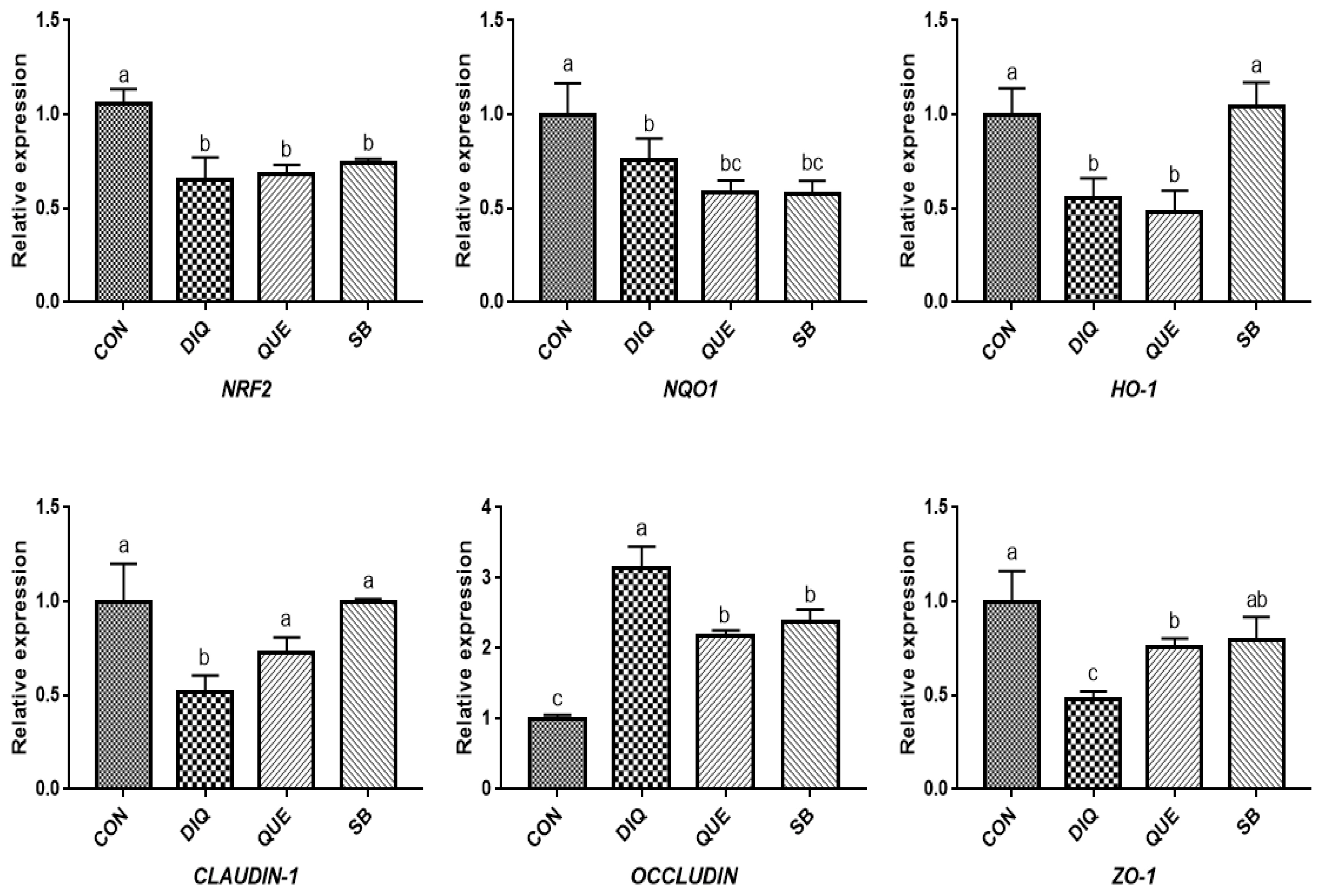
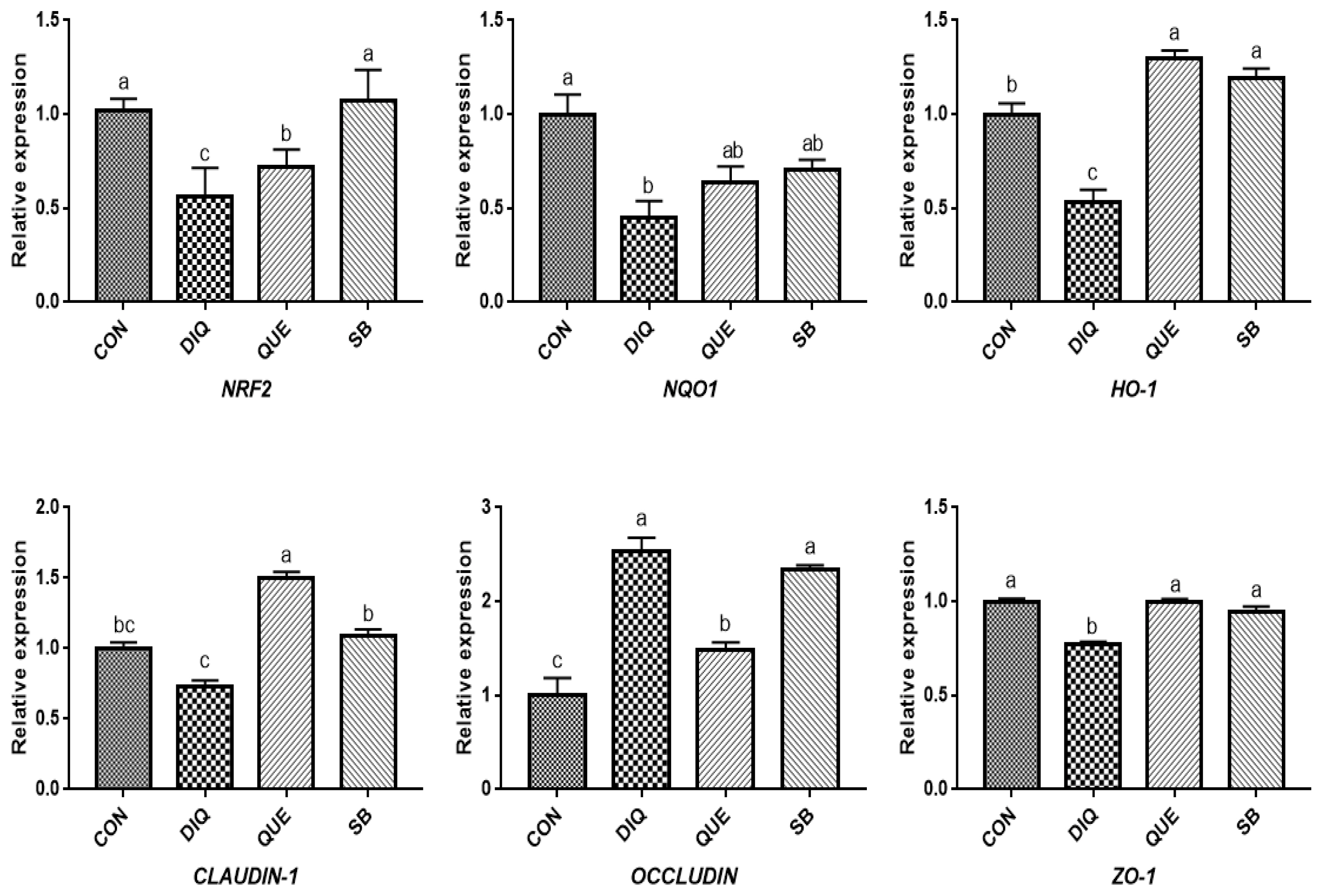
NRF2, a pivotal sensor of oxidative stress, is a transcription factor that plays a central role in the regulation of antioxidant and phase 2 detoxifying enzymes and related proteins, and has been reported to regulate the antioxidant response and represent the underlying mechanism that provides a pivotal defense against DIQ toxicity [
25]. Once stimulated by inducers, NRF2 activates downstream enzymes, including NQO1 and HO-1, to prevent oxidative stress damage. In the present study, DIQ treatment caused downregulated intestinal
NRF2 expression and its downstream target genes
NQO1 HO-1, which can be considered an acute response to DIQ-induced oxidative stress, which is in agreement with previous results [
24,
26]. Previous studies have shown that supplementation with QUE attenuates the lipopolysaccharide-induced reduction of
HO-1 and
NQO1 mRNA levels in jejunum tissue [
14] and activates the NRF2 signaling pathway to induce HO-1 expression and protect against oxidative stress [
12]. Additionally, QUE has been found to effectively inhibit manganese-induced oxidative stress in neural-tumor epithelial cells (SK-N-MC) in Sprague-Dawley rats by upregulating HO-1 and Nrf2 proteins [
27]. Our findings showed that QUE promoted
NRF2 and
HO-1 expression levels in the jejunum and ileum on the tenth day compared with the DIQ group, which is consistent with previous findings, suggesting that QUE inhibited DIQ-induced intestinal oxidative stress through the NRF2 signaling pathway. In the present study, we observed upregulation of
NRF2 in the ileum and
NQO1 on the tenth day, which is similar to a previous study in which the mRNA expression of
NQO1 and
NRF2, as well as total NRF2 protein levels, were increased in goats fed a sodium butyrate diet [
28]. The intestinal barrier is physically composed of epithelial cells connected by tight junction proteins, such as ZO-1, CLAUDIN-1, and OCCLUDIN, and regulation of the selective permeability between epithelial cells and gene expression is important for maintaining the proper functioning of the intestinal epithelial barrier [
29]. Tight junction mRNAs and protein expressions varied in response to DIQ exposure. Diquat-induced oxidative stress causes a decrease in tight junction genes expression, leading to increased intestinal permeability, weakening intestinal barrier function and disrupting intestinal structural integrity the structural integrity of the intestine [
7,
8]. Diquat treatment decreased protein levels of OCCLUDIN, CLAUDIN-1, and ZO-1 in the jejunal mucosa [
7]. However, this study found that the mRNA expression of
OCCLUDIN was significantly increased and that
CLAUDIN-1 and
ZO-1 levels were significantly decreased in the jejunum and ileum of the DIQ group. Whether this phenomenon was induced by DIQ still requires further investigation and may be related to a compensatory effect. Quercetin improved gut barrier function, the QUE-supplemented showed increased
OCCLUDIN and
ZO-1 mRNA expression in the jejunum of pigs after transport stress [
22], different stress patterns may account for differential expression of
CLAUDIN-1 and
OCCLUDIN. One report have investigated the effect of sodium butyrate on tight junction proteins, and the mRNA and protein expression of CLAUDIN-1 and ZO-1 in goat ruminal epithelium increased after sodium butyrate supplementation, suggested that sodium butyrate could reverse the damage of rumen epithelium tight junction by inhibiting MAPK signaling pathways [
30]. Differences of related genes expression between jejunum and ileum may be due to additives working in different parts of the small intestine [
26]. Collectively, the present findings provide important evidence of the potential protective effects of QUE and coated sodium butyrate against DIQ-induced dysfunction in the intestinal morphology and barrier of pullets.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





