 |
 |
| Anim Biosci > Volume 35(8); 2022 > Article |
|
Abstract
Objective
The objective of this study was to investigate the in vitro fermentation profiles of different soybean oligosaccharides (SBOs) and their effects on skatole production and cecal microbiota of broilers.
Methods
Five SBOs with varying main component contents were fermented using an in vitro batch incubation inoculated with broiler cecal microbiota. Gas production was recorded automatically, skatole, indole and short-chain fatty acids (SCFAs) were determined using high-performance liquid chromatography, and microbial changes were analyzed using 16S DNA gene sequencing.
Results
The addition of SBOs increased (p<0.05) gas production, suggesting bacterial growth-stimulating activities. In addition, the concentrations of indole were significantly (p<0.05) decreased after SBO supplementation, and SBO III, with higher sucrose and stachyose contents, decreased (p<0.05) the skatole level. Our results also revealed that the fermentation of SBOs by cecal microbiota produced (p<0.05) SCFAs, which were dominated by propionic acid, butyrate acid and lactic acid compared to the control. In addition, SBO III increased (p<0.05) the abundance of Firmicutes and Subdoligranulum and decreased that of Bacteroides.
Soybean oligosaccharides (SBOs) are a group of soluble oligosaccharides found in soy or other legumes that consist primarily of stachyose, raffinose, and sucrose [1]. SBOs are not usually digested by human or animal digestive enzymes of the upper gastrointestinal tract but are instead selectively fermented by some types of intestinal bacteria in the lower tract [2]. This selective stimulation of a limited number of bacteria affects the host by modulating the microbial community composition and metabolites, such as short-chain fatty acids (SCFAs), in a presumably beneficial way [3]. Dietary supplementation with SBOs has been demonstrated to have prebiotic effects on growth performance, immune modulation and intestinal microbial communities in pigs and broilers [4].
Recently, SBOs were shown to have the ability to lower skatole production [5,6]. Skatole is one of the most noticeable fecal odorants and has a detectable threshold of 0.003 mg/m3, thereby having an adverse influence on the productivity and welfare of intensively fed animals [7]. The hindgut is the main site where skatole and indole are mainly produced by the microbial degradation of L-tryptophan (L-Trp) in the diet [8,9]. Previous studies have demonstrated that the addition of SBOs can decrease the rate of L-Trp degradation and the concentration of indole and skatole in vitro, in the cecum, and in excreta [5,6,10]. However, the differences in the source and production technology lead to the difference in the spatial structure, thus resulting in the difference in the prebiotic activity of SBOs. Therefore, in this study, an in vitro fermentation model was used to investigate the effects of different SBOs with varying main component contents on skatole production and the cecal microbiota of broilers, and the results may contribute to evaluating the prebiotic potential of SBOs to modulate gut health and reduce odor emission in broiler production.
All procedures involving animals were reviewed and approved by the Animal Care and Use Committee of Shenyang Agricultural University (201806010).
The SBOs used in the present study were commercially available and supplied by Henan Tiandi Biotech Co Ltd. (SBO I); Henan Fenglu Biotech Co Ltd. (SBO II); Shanxi Jinrun Biotech Co Ltd. (SBO III); XiŌĆÖan Baichuan Biotech Co Ltd. (SBO IV); and Guangdong Yibaolai Biotech Co Ltd. (SBO V), China. The functional ingredients in SBOs are sucrose, raffinose and stachyose, and the contents of total sugar (%), sucrose, raffinose and stachyose (mg/g) of SBOs used in this study were determined and shown below: SBO I (66.50%, 66.98, 100.75, 549.79), SBO II (82.75%, 61.82, 114.29, 509.02), SBO III (75.53%, 70.12, 84.70, 559.70), SBO IV (65.54%, 60.36, 109.98, 520.51), and SBO V (70.39%, 77.24, 84.56, 641.44). The moisture content was all less than 5%.
Twenty Arbor Acres one-day-old commercial straight run broiler chicks with an average initial body weight were allocated randomly into 2 cages of 10 birds each (male and female in half). Cages equipped with a separate feeder and a nipple drinker from 1 d to 49 d. The basal soybean meal-free diet was formulated to exclude the interference factor of oligosaccharides from soybeans and to meet the nutrient requirements recommended by the Arbor Acres management guide (Table 1). On day 49, broiler chicks were sacrificed, abdominal cavities were opened immediately, and both ceca of each chick were collected aseptically, tied from open sides, placed into empty sterile plastic bags individually and stored at ŌłÆ80┬░C.
A single factor complete random design was adopt in this study. A total of six treatments were included: one control and five SBO treatments (SBO I to SBO V). The total volume of the fermentation broth was 200 mL each, and 1.5% SBO was contained in a treatment on a total sugar basis and L-Trp was added to each treatment at a final concentration of 250 ╬╝mol/L. During each incubation, no L-Trp addition was used for adjustment for gas and fermentation metabolite production. The incubation temperature and time were 41┬░C and 24 h, respectively. Each treatment was carried out in 3 technical repetitions.
The basal salt medium was formulated according to Wang et al [11] and then autoclaved (121┬░C, 15 min) for sterilization [11]. The mixed cecal digesta (male:female = 1:1) were suspended under a constant flow of CO2 gas in sterile basal salt medium to give a final concentration of 100 g of digesta/L and homogenized in a blender. A volume of 198 mL of the suspension was added to a fermenter autoclaved before use, and 2 mL of L-Trp solution was added to give a final concentration of 250 ╬╝mol/L. As a control, a fermenter with 198 mL of the suspension and 2 mL of sterilized distilled water was used to measure the cecal contribution to the production of gas, skatole, indole, and SCFA. The incubation was kept at 41┬░C for 24 h. Samples (10 mL) were taken after incubation for analysis.
The ANKOM-RFS gas production automated system (GPM 9.8.1) (ANKOM Technology, Macedon, NY, USA) was used to measure gas production following the equation:
where Vxt (mL) is the cumulative gas production at time t (h) during fermentation, Vj (mL) is the headspace of the glass bottles, Ppsit is the cumulative pressure (psi) of the sample modules and Ppsi0 is the psi of the blank module at time t as recorded by the system.
The skatole and indole concentrations were analyzed by the high-performance liquid chromatography (HPLC) method as described previously [10]. The HPLC conditions were as follows: column, 250 mm├Ś4.6 mm├Ś5 ╬╝m (Dikma, Beijing, China); eluent, acetonitrile/water (40:60, v/v); flow, 1.0 mL/min; excitation wavelength, 263 nm; emission wavelength, 358 nm; injection volume, 20 ╬╝L.
The volatile fatty acid and lactic acid concentrations were analyzed by HPLC method as described previously [10]. The HPLC conditions were as follows: column, 250 mm├Ś4.6 mm├Ś 5 ╬╝m (Dikma, China); eluent, phosphate buffer (pH 2.5)/methyl alcohol (95:5, v/v); flow, 1.0 mL/min; injection volume, 10 ╬╝L.
Total genomic DNA from the fermentation broth samples was extracted using a Qubit 2.0 DNA kit according to the manufacturerŌĆÖs instructions. 16S rDNA genes of V3ŌĆōV4 were amplified using 341F-805R (341F: 5ŌĆ▓-CCCTACACGACGC TCTTCCGATCTG-3ŌĆ▓; 805R: 5ŌĆ▓-GACTGGAGTTCCTTG GCACCCGAGAATTCCA-3ŌĆ▓) with the barcode as described by Liu et al [6]. The polymerase chain reaction (PCR)-amplification products were verified by agarose gel electrophoresis (1.5% agarose).
A total of 18 cecal fermentation samples were collected and sent for sequencing. Raw reads were quality filtered to remove primer connector sequences, nonamplification sequences, or chimeric sequences [12]. The remaining high-quality reads with sequence lengths of 400 to 440 bp across all the samples were processed, merged and clustered into operational taxonomic units based on a 97% sequence similarity threshold. The species annotation and statistical analysis of the species composition for each sample at the phylum and genus levels were performed by the Ribosomal Database Project classifier software and the Greengenes database [13]. Microbial community structure analysis was included for the observed species and the alpha diversity indices Chao1 index and ACE index, which were used to estimate species richness, Shannon index and Simpson index, were used to estimate species diversity [14]. Principal coordinates analysis and cecal microbiota cluster trees, which revealed the similarity measure of bacterial community based on phylogenetic distance, were performed based on unweighted UniFrac distance matrices.
Statistical analysis was performed by one-way analysis of variance using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA), and significant differences among treatments were compared using DuncanŌĆÖs multiple comparison tests [10]. Differences with p-values less than 0.05 were considered statistically significant.
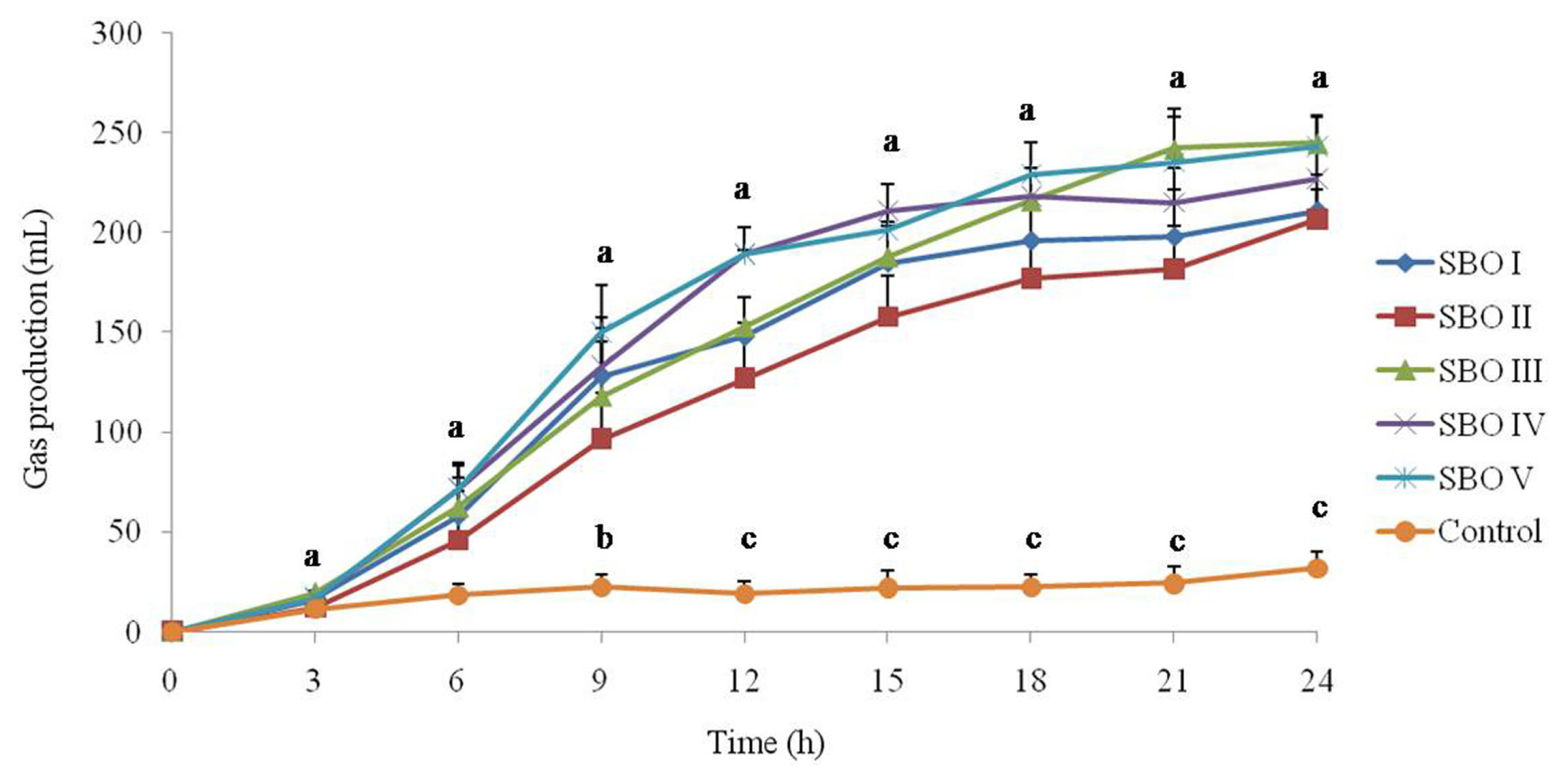
The cumulative gas production is shown in Figure 1. All tested SBOs produced gas when they were utilized by cecal microbial communities. Gas production in the control was low at all sampling times, while the addition of SBOs increased (p<0.05) gas production after 9 h of incubation and even more significant (p<0.01) after 12 h of incubation. Among SBOs, SBO II had a lower (p<0.05) cumulative gas production than SBOs IV and V after 12 h of incubation.
Skatole concentrations showed no significant difference (p> 0.05) among treatments except SBO III, which had a lower (p<0.01) skatole level. Compared to the control, indole concentrations of SBOs were lower (p<0.01) (shown in Figure 2).
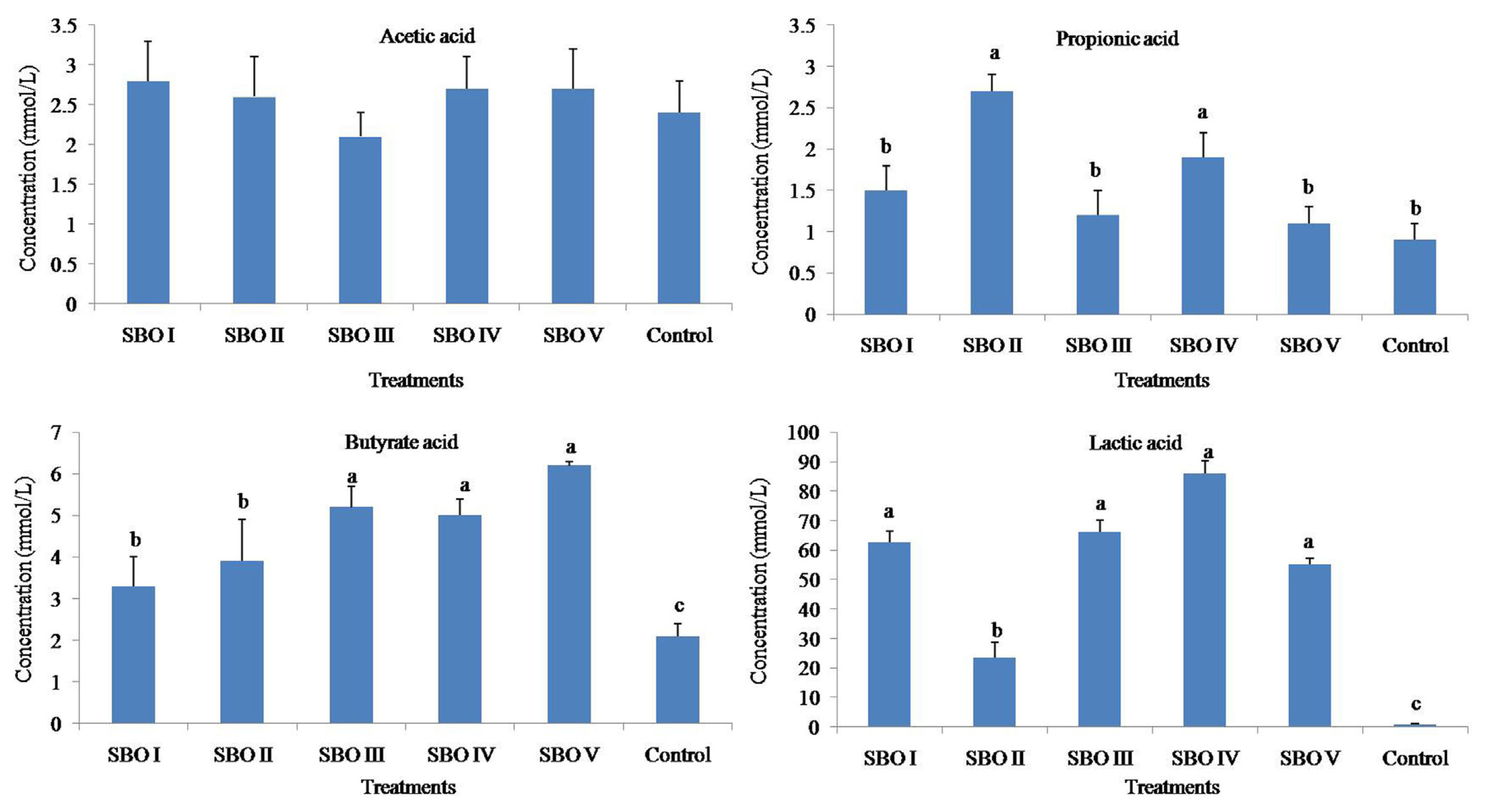
The addition of SBOs increased (p<0.01) the concentrations of propionic acid, butyrate acid and lactic acid but did not (p>0.05) affect that of acetic acid. The butyrate acid concentrations in SBOs III, IV, and V were higher than those in SBOs I and II (shown in Figure 3).
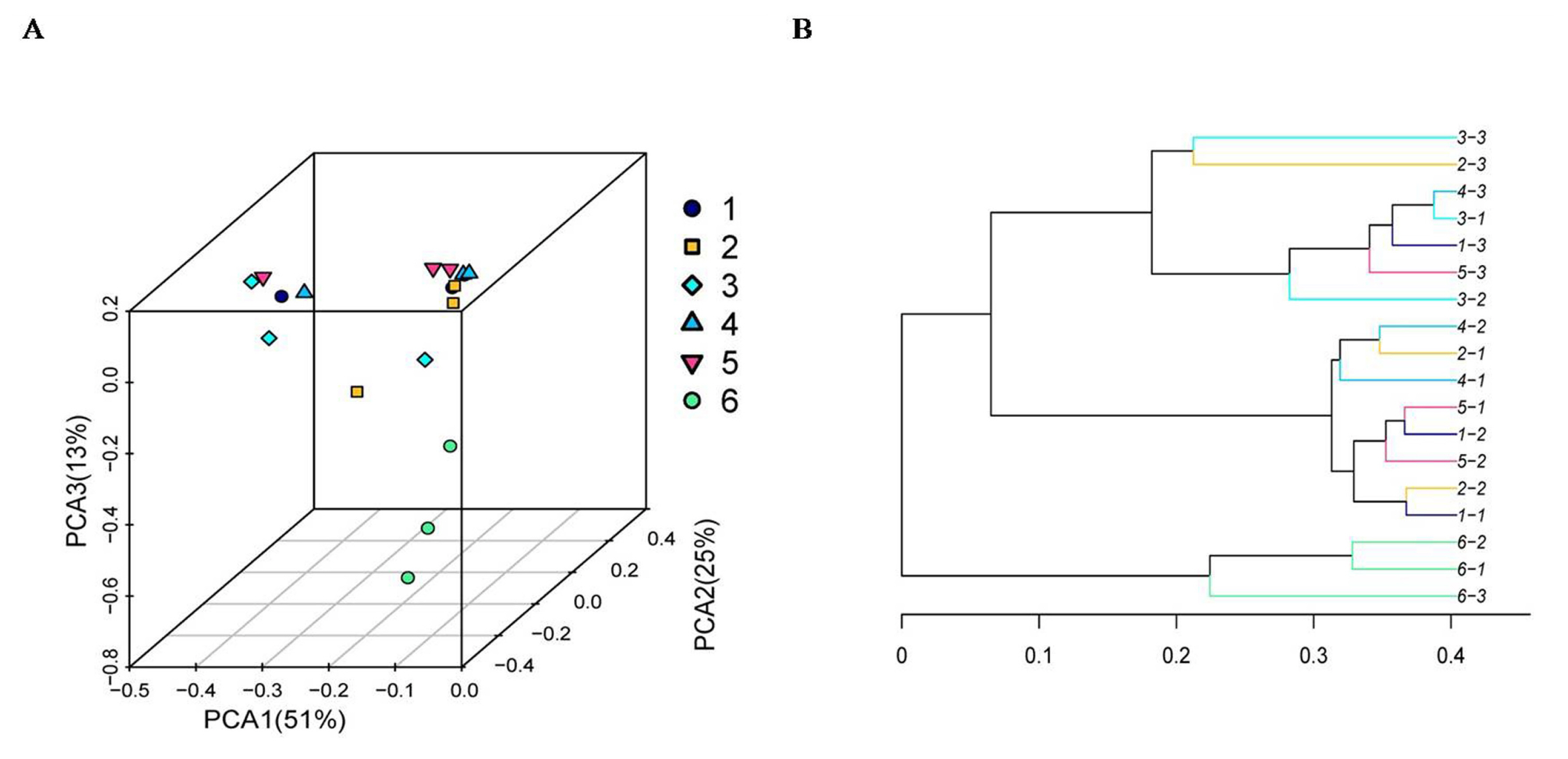
As shown in Figure 4, the Chao 1 index and ACE index of the SBO treatments were higher (p<0.01) than those of the control. However, there was no statistical difference (p> 0.05) in the Shannon index and the Simpson index among treatments, although the Shannon index of the control was numerically higher than that of the SBO groups. The results of the cluster trees showed a degree of diversity discrepancy between the SBO III group and the control, while the samples from other SBO treatments clustered together (Figure 5).
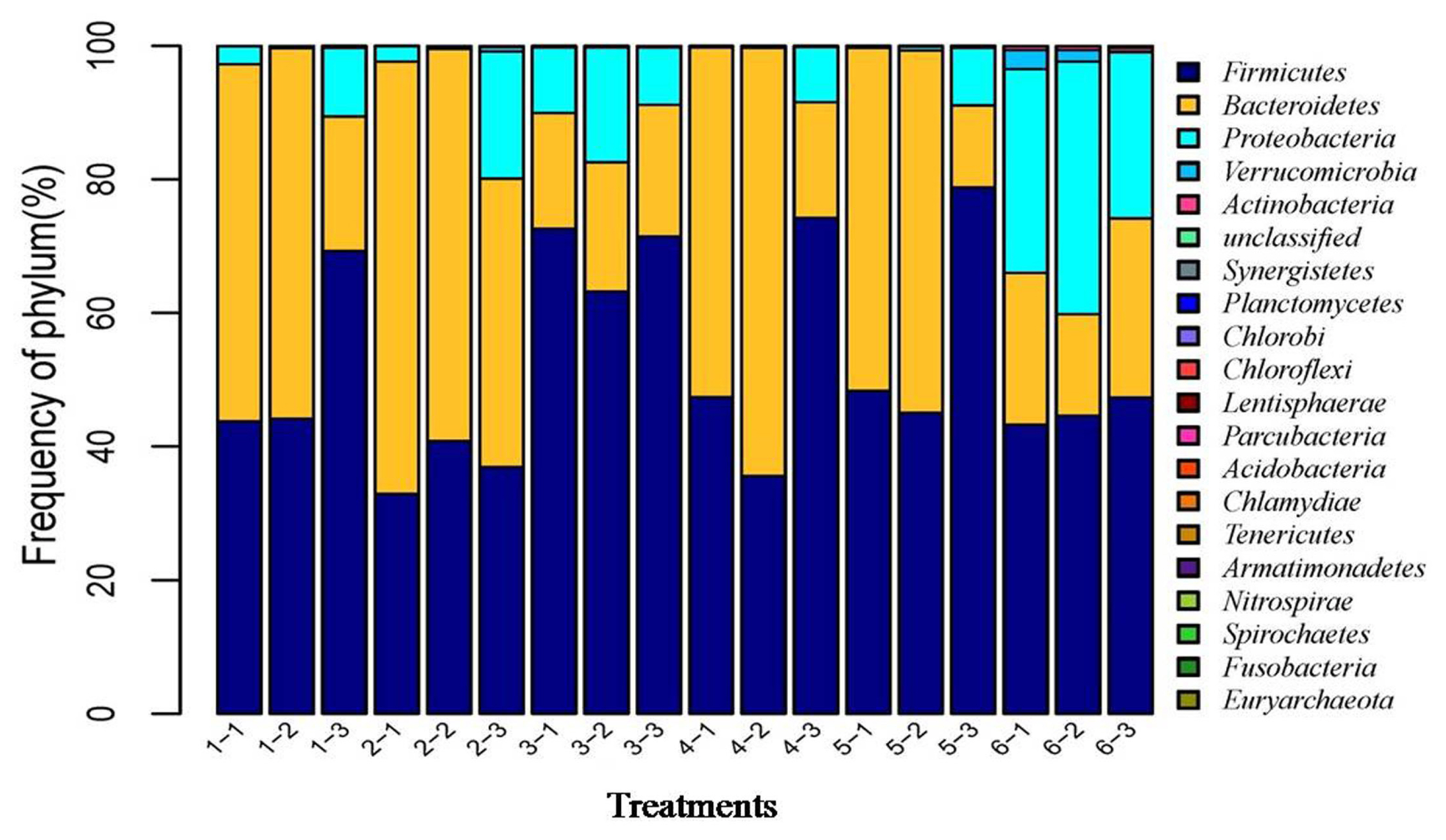
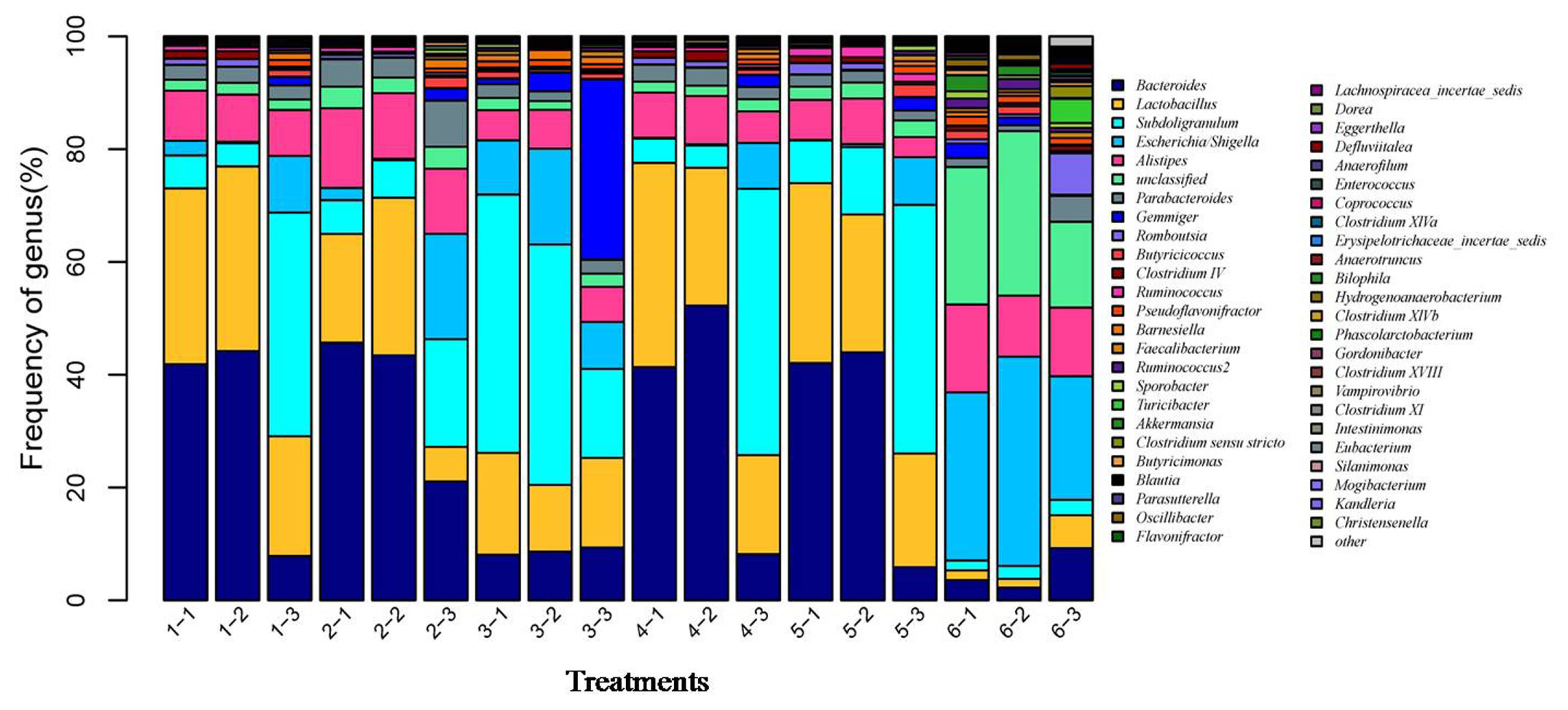
After the microbial diversity analysis, the taxonomic profiles of the fermentation samples were explored. As shown in Figure 6 and 7, seven phyla and twenty-three genera with relative abundances greater than 0.1% were present. At the phylum level, Firmicutes and Bacteroidetes were prevalent among all treatments but were different in the control and SBO treatments. It is worth noting that Bacteroidetes were decreased in SBO III compared with the other SBO treatments, whereas Proteobacteria increased (p<0.01). At the genus level, Bacteroides and Lactobacillus were present in all treatments. Compared with the control, the addition of SBOs decreased the abundance of Escherichia/Shigella, Alistipes, Sporabacter, Ruminococcus2 and Butyricumonas and increased that of Ruminococcus. The results also showed that Bacteroides and Ruminococcus were less abundant and Subdoligranulum was more abundant in SBO III than in the other SBO treatments.
Odorous compounds from livestock and poultry systems are mainly produced from the microbial fermentation of undigested nutrients of the diet in the gut [15]. In poultry, skatole and indole are produced from a multistep degradation of L-Trp by microbial activity and are considered to be contributors to excreta malodor [16]. In addition, the concentrations of skatole and indole in the cecum are significantly higher than those in the other gut segments [9]. Therefore, the cecal digesta of broilers were collected and used as the fermentation medium to determine skatole and indole production in this study.
Oligosaccharides have been reported to possess prebiotic activities and can be utilized by the cecal microbial communities of broiler chickens [17]. During the fermentation process, oligosaccharides can be fermented to produce gases and SCFAs, which are ensemble products of the activities of the total microbiota present in fermentation [2,18]. Thus, gas and SCFA production are one of the most visual indices to evaluate the fermentation state and microbial activity. Along with gas production, SCFAs, including acetic acid, propionic acid, butyric acid and lactic acid, are also produced, which provide important carbon sources for microbial protein synthesis and energy for intestinal cells [3,19,20]. The cumulative gas and SCFA production results in the present study showed that the addition of SBOs had a strong ability to increase gas and SCFA production, implying that SBOs were much easier to utilize by cecal microbes of broiler chickens. In addition, we also observed that fermentation of different SBOs gave rise to varied gas and SCFA production. Presumably, the reason for this may be the varied content and composition of components in SBOs. As the main components of SBOs, raffinose contains fructose, glucose, and galactose, while stachyose contains one more galactose [1]. Different SBOs with varied degrees of polymerization can cause varied production of gases and SCFAs and fermentation profiles. Lan et al [2] compared in vitro gas production for SBO, stachyose and raffinose and found that stachyose had higher gas production and maximum rate of gas production than SBO and raffinose [2]. Similarly, in this study, SBOs with a lower content of stachyose (such as SBO II) had a lower gas production, implying that stachyose was a key component of SBOs to be correlated with gas production. In addition, Lan et al [2] also found that stachyose had a stronger ability to induce propionic acid production, but not acetic acid or butytic acid, than raffinose in vitro fermentation. Zhu et al [10] also found that dietary supplementation with 0.6% stachyose significantly increased the propionic acid concentration in the cecum of broilers, and 0.6% raffinose seemed to contribute to butyric acid and lactic acid production. Thus, the addition of SBOs can increase the amount of fermenable carbohydrates, stimulate the growth of specific bacteria, and lead to gas and SCFA production.
Skatole is a well-known, foul-smelling fecal odorant affecting the production and welfare of farm animals [7]. Dietary supplementation with some oligosaccharides, such as SBOs, contributes to the modulation of intestinal microbiota by simulating the proliferation of beneficial strains and inhibiting the growth of pathogenic bacteria and then reduces the production of odor compounds [5,10,21]. Liu et al [6] found that the in vitro addition of SBO to fermentation broth at a content of 10 g/L total sugar decreased the rate of L-Trp degradation and the concentration of skatole and indole. The components of SBOs, such as stachyose and raffinose, have been demonstrated to affect the production of skatole and indole. Zhu et al [10] also demonstrated that dietary supplementation with 0.6% stachyose significantly decreased the cecal concentration of skatole compared with raffinose in broilers. The results from Liu et al [22] further demonstrated that broiler cecal skatole levels can be influenced by dietary stachyose levels, and 5 g/kg stachyose in the diet was suggested. Similarly, in this study, SBOs with higher sucrose and stachyose contents had a stronger ability to lower skatole and indole production, suggesting that the addition of SBOs may stimulate the growth of carbohydrate-degrading bacteria, not protein-degrading bacteria, and inhibit protein decomposition, thus decreasing the L-Trp flow supply and resulting in reduced skatole and indole production.
In general, the intestinal microbiota of the host contains trillions of symbiotic microorganisms and remains quite stable once established [23,24]. There are many factors, including diet, breed type, and housing environment, which can affect the gut microbiota [25,26]. Of these, diet is the principal environmental factor that can directly influence the nature of the microbiota in the host. Previous studies have demonstrated that dietary supplementation with prebiotics, such as SBOs, which have received much attention after phasing out antibiotic growth promoters in poultry feed, can be selectively fermented and has been linked to the selective growth promotion of probiotics, chiefly Bifidobacterium and Lactobacillus, thus offering competitive colonization resistance against pathogenic bacteria [5,10,27]. Previous studies have demonstrated that in vitro or dietary addition of oligosaccharides resulted in an increase in microbial diversity and species richness [4,21,22]. Similarly, our data also showed that the addition of SBOs increased the microbial diversity and species richness in fermentation broth, indicative of stimulating the growth of microorganisms by SBOs. Significant differences in the populations of bacteria at the phylum level were observed. As a dominant bacterial phylum in the gut microbiota, Firmicutes are always abundant in the lower gut and are capable of degrading oligosaccharides into SCFAs [28,29]. For Bacteroidetes, which are generally considered primary saccharolytic bacteria capable of degrading an extensive array of complex carbohydrates [30], the relative abundance in SBOs was higher than that in the control. At the genus level, Lactobacillus were strongly stimulated to proliferate by the addition of SBOs, in agreement with previous results showing an increase in Lactobacillus in broilers fed SBOs [5]. Although all SBOs can be degraded by complex gut microbiota, the emergence of high relative abundance bacteria in different SBOs varied. Bacteroides was less abundant in SBO III than in other SBO treatments and correlated with reduced skatole production, which is in agreement with previous results showing that Bacteroides can metabolize tryptophan to indole-3-lactate and then to indole and skatole [10].
In conclusion, the present work provides evidence on the in vitro prebiotic potential of the different SBOs and can be beneficial to gut health. The results indicated that the addition of SBOs can selectively stimulate the growth and activity of beneficial bacteria, increase propionic acid, butyrate acid and lactic acid levels and reduce skatole and indole production in broilers. Moreover, the microbial community structure was substrate-dependent with SBOs, and SBO III, with higher sucrose and stachyose contents, markedly impacted the relative abundance of Bacteroides and Subdoligranulum. This finding might point out a potential relationship between the main components of SBOs and their impact on the gut microbiota composition and odor compound production.
Notes
Figure┬Ā1
Effect of different soybean oligosaccharides (SBOs) on gas production in cecal fermentation broth from broilers. Different letters indicate significant differences among treatments (p<0.05).
Figure┬Ā2
Effects of different soybean oligosaccharides on indole and skatole concentrations in cecal fermentation broth from broilers. Different letters indicate significant differences among treatments (p<0.05).

Figure┬Ā3
Effects of different soybean oligosaccharides (SBOs) on SCFA concentrations in cecal fermentation broth from broilers. Different letters indicate significant differences among treatments (p<0.05).
Figure┬Ā4
Alpha diversity index of cecal microbiota in fermentation broth from broilers. A, Simpson index; B, Chao1 index; C, Shannon index; D, ACE index. Treatment 1 to 5, SBO 1 to 5; Treatment 6, control.

Figure┬Ā5
Principal coordinate analysis (A) and cluster tree diagram (B) of cecal microbiota in fermentation broth from broilers. Treatment 1 to 5, SBO 1 to 5; Treatment 6, control.
Figure┬Ā6
Effects of different soybean oligosaccharides (SBOs) on cecal microbiota in fermentation broth at the phylum level. Treatment 1 to 5, SBO 1 to 5; Treatment 6, control.
Figure┬Ā7
Effects of different soybean oligosaccharides (SBOs) on cecal microbiota in fermentation broth at the genus level. Treatment 1 to 5, SBO 1 to 5; Treatment 6, control.
Table┬Ā1
Ingredient and chemical composition of the basal diet (air dry basis, %)
| Items | Content | |
|---|---|---|
|
|
||
| 0 to 3 wk | 4 to 7 wk | |
| Ingredients | ||
| ŌĆāCorn | 41.40 | 47.32 |
| ŌĆāCorn umbilicus pulp | 23.36 | 16.00 |
| ŌĆāCorn protein | 18.70 | 15.16 |
| ŌĆāSoybean oil | 3.54 | 4.34 |
| ŌĆāCorn DDGS | 3.00 | 5.00 |
| ŌĆāRice bran | 5.00 | 8.00 |
| ŌĆāLimestone | 1.20 | 1.14 |
| ŌĆāCaHPO4 | 1.42 | 1.05 |
| ŌĆāNaCl | 0.26 | 0.27 |
| ŌĆāLys | 1.00 | 0.84 |
| ŌĆāMet | 0.24 | 0.20 |
| ŌĆāThr | 0.22 | 0.17 |
| ŌĆāArg | 0.45 | 0.30 |
| ŌĆāPhytase | 0.01 | 0.01 |
| ŌĆāCholine chloride | 0.05 | 0.05 |
| ŌĆāVitamin mix1) | 0.05 | 0.05 |
| ŌĆāMineral mix2) | 0.10 | 0.10 |
| ŌĆāTotal | 100.00 | 100.00 |
| Calculated values | ||
| ŌĆāME (MJ/kg) | 12.77 | 13.39 |
| ŌĆāCP | 22.00 | 19.50 |
| ŌĆāEE | 6.89 | 8.31 |
| ŌĆāCa | 0.92 | 0.79 |
| ŌĆāTotal P | 0.67 | 0.62 |
| ŌĆāAvailable P | 0.36 | 0.30 |
| ŌĆāAvailable Lys | 1.30 | 1.12 |
| ŌĆāAvailable Met | 0.65 | 0.57 |
| ŌĆāAvailable Met+Cys | 0.90 | 0.80 |
| ŌĆāAvailable Thr | 0.93 | 0.80 |
DDGS, dry distillers grains with solubles; ME, metabolizable energy; CP, crude protein; EE, ether extract; Ca, calcium; P, phosphorus.
REFERENCES
1. Obendorf R, Horbowicz M, Dickerman AM, Brenac P, Smith ME. Soluble oligosaccharides and galactosyl cyclitols in maturing soybean seeds in planta and in vitro. Crop Sci 1998; 38:78ŌĆō84.
https://doi.org/10.2135/cropsci1998.0011183X003800010014x

2. Lan Y, Willianms BA, Verstegen MWA, Patterson R, Tamminga S. Soy oligosaccharides in vitro fermentation characteristics and its effect on caecal microorganisms of young broiler chickens. Anim Feed Sci Technol 2007; 133:286ŌĆō97.
https://doi.org/10.1016/j.anifeedsci.2006.04.011

3. Mahmood T, Guo Y. Dietary fiber and chicken microbiome interaction: Where will it lead to? Anim Nutr 2020; 6:1ŌĆō8.
https://doi.org/10.1016/j.aninu.2019.11.004



4. Zhou XL, Kong XF, Lian GQ, Blachier F, Geng MM, Yin YL. Dietary supplementation with soybean oligosaccharides increases short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal content of weaned Huanjiang mini-piglets. Nutr Res 2014; 24:780ŌĆō8.
https://doi.org/10.1016/j.nutres.2014.08.008

5. Yang GQ, Yin Y, Liu HY, Liu GH. Effects of dietary oligosaccharide supplementation on growth performance, concentrations of the major odor-causing compounds in excreta, and the cecal microflora of broilers. Poult Sci 2016; 95:2342ŌĆō51.
https://doi.org/10.3382/ps/pew124


6. Liu HY, Hou R, Yang GQ, Zhao F, Dong WG. In vitro effects of inulin and soya bean oligosaccharide on skatole production and the intestinal microbiota in broilers. J Anim Physiol An Nutr 2018; 102:706ŌĆō16.
https://doi.org/10.1111/jpn.12830

7. Deslandes B, Gariepy C, Houde A. Review of microbiological and biochemical effects of skatole on animal production. Livest Prod Sci 2001; 71:193ŌĆō200.
https://doi.org/10.1016/S0301-6226(01)00189-0

8. ├śverland M, Kjos NK, Fauske AK, Teige J, S├Ėrum H. Easily fermentable carbohydrates reduce skatole formation in the distal intestine of entire male pigs. Livest Sci 2011; 140:206ŌĆō17.
https://doi.org/10.1016/j.livsci.2011.03.032

9. Yang GQ, Zhang P, Liu HY, Zhu X, Dong WG. Spatial variations in intestinal skatole production and microbial composition in broilers. Anim Sci J 2019; 90:412ŌĆō22.
https://doi.org/10.1111/asj.13164


10. Zhu X, Liu JZ, Liu HY, Yang GQ. Soybean oligosaccharide, stachyose, and raffinose in broilers diets: effects on odor compound concentration and microbiota in cecal digesta. Poult Sci 2020; 99:3532ŌĆō9.
https://doi.org/10.1016/j.psj.2020.03.034



11. Wang JF, Zhu YH, Li DF, Wang Z, Jensen BB. In vitro fermentation of various fiber and starch sources by pig fecal inocula. J Anim Sci 2004; 82:2615ŌĆō22.
https://doi.org/10.2527/2004.8292615x


12. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011; 27:2194ŌĆō200.
https://doi.org/10.1093/bioinformatics/btr381



13. Wang Q, Garrity GM, Tiedje JM, Cole JR. Na├»ve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007; 73:5261ŌĆō7.
https://doi.org/10.1128/AEM.00062-07



14. Qiao H, Song Y, Shi H, Bian C. Fermented Astragalus in diet altered the composition of fecal microbiota in broiler chickens. AMB Express 2018; 8:151
https://doi.org/10.1186/s13568-018-0682-4



15. Feng J, Lu M, Wang J, et al. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J Anim Sci Biotechnol 2021; 12:72
https://doi.org/10.1186/s40104-021-00600-3



16. Wang YC, Han MF, Jia TP, et al. Emissions, measurement, and control of odor in livestock farms: A review. Sci Total Environ 2021; 776:145735
https://doi.org/10.1016/j.scitotenv.2021.145735


17. Azad MAK, Gao J, Ma J, et al. Opportunities of prebiotics for the intestinal health of monogastric animals. Anim Nutr 2020; 6:379ŌĆō88.
https://doi.org/10.1016/j.aninu.2020.08.001



18. Lan Y, Williams BA, Tamminga S, et al. In vitro fermentation kinetics of some non-digestible carbohydrates by the caecal microbial community of broilers. Anim Feed Sci Technol 2005; 123ŌĆō4:687ŌĆō702.
https://doi.org/10.1016/j.anifeedsci.2005.04.027

19. Wang G, Huang S, Wang Y, et al. Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol Life Sci 2019; 76:3917ŌĆō37.
https://doi.org/10.1007/s00018-019-03190-6



20. Zhou H, Yu B, He J, et al. The optimal combination of dietary starch, non-starch polysaccharides, and mannan-oligosaccharide increases the growth performance and improves butyrate-producing bacteria of weaned pigs. Animals 2020; 10:1745
https://doi.org/10.3390/ani10101745



21. Patel S, Goyal A. Functional oligosaccharides: production, properties and applications. World J Microbiol Biotechnol 2011; 27:1119ŌĆō28.
https://doi.org/10.1007/s11274-010-0558-5

22. Liu HY, Zhao XY, Yang GQ, Liu JZ, Zhu X. Effects of dietary stachyose levels on caecal skatole concentration, hepatic cytochrome P450 mRNA expressions and enzymatic activities in broilers. Br J Nutr 2020; 124:1013ŌĆō20.
https://doi.org/10.1017/S0007114520002263


23. Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 2018; 57:1ŌĆō24.
https://doi.org/10.1007/s00394-017-1445-8



24. Wang H, Ren P, Mang L, Shen N, Chen J, Zhang Y. In vitro fermentation of novel microwave-synthesized non-digestible oligosaccharides and their impact on the composition and metabolites of human gut microbiota. J Funct Foods 2019; 55:156ŌĆō66.
https://doi.org/10.1016/j.jff.2019.02.030

25. Borda-Molina D, Seifert J, Camarinha-Silva A. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput Struct Biotechnol J 2018; 16:131ŌĆō9.
https://doi.org/10.1016/j.csbj.2018.03.002



26. Feye KM, Baxter MFA, Tellez-Isaias G, Kogut MH, Riche SC. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult Sci 2020; 99:653ŌĆō9.
https://doi.org/10.1016/j.psj.2019.12.013



27. Liu HY, Li X, Zhu X, Dong WG, Yang GQ. Soybean oligosaccharides attenuate odour compounds in excreta by modulating the caecal microbiota in broilers. Animal 2021; 15:100159
https://doi.org/10.1016/j.animal.2020.100159


28. Choi KY, Lee TK, Sul WJ. Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens-a review. Asian-Australas J Anim Sci 2015; 28:1217ŌĆō25.
https://doi.org/10.5713/ajas.15.0026



29. Xiao Y, Xiang Y, Zhou W, Chen J, Li K, Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult Sci 2017; 96:1387ŌĆō93.
https://doi.org/10.3382/ps/pew372


30. Martens EC, Lowe EC, Chiang H, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 2011; 9:e1001221
https://doi.org/10.1371/journal.pbio.1001221









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





