 |
 |
| Anim Biosci > Volume 34(2); 2021 > Article |
|
Abstract
Objective
The present study evaluated the preservation of ram semen at 0°C using soybean lecithin with a Tris-fructose extender.
Methods
Semen was collected by artificial vagina ejaculation from six rams with proven fertility. High quality ejaculates were diluted by soybean lecithin (0.25%, 0.5%, 0.75%, 1.0%, 1.25%) using Tris-fructose extender and control (Tris-fructose egg yolk extender), respectively. The ejaculates were diluted to a concentration of 5×108 sperm/mL, followed by cooling to 0°C in 90 min and maintaining the temperature for 12 days. The diluted semen samples were examined and recorded for sperm progressive motility, acrosome integrity at 0, 24, 72, 144, 216, 288 h, respectively. Two hundred and twenty-three ewes were inseminated for 216 h with optimal soybean lecithin concentrated semen or control via trans-cervical insemination.
Results
The results showed that there were no differences in sperm progressive motility at 0, 24, 72, and 144 h (p>0.05). After 216 h, the sperm progressive motility in the control group and 0.5% concentration groups was significantly higher when compared to 0.25% concentration (p<0.05). The 0.5% concentration group demonstrated the highest survival rate and had no difference with the control group (p>0.05). At 216 h, the sperm progressive motility of all groups was still above 50%. The acrosome integrity of all groups was decreased with prolongation of storage time, but there was no difference at each time point (p>0.05). There was no significant difference in the lambing rate and pregnancy rate between the 0.5% concentration group and the control group (p>0.05).
Long-term preservation of sperm in the field of animal and human medicine is regarded as an important approach for enhancing reproductive technology [1]. Since the invention of freezing technology, the sheep sperm freezing technology has not made substantial progress, and the reason for this is that the sheep sperm is vulnerable to cold shock, and cryopreservation of the sperm seriously reduces the motility of the sperm. The membrane function and the biology of sperm cells are affected, which thus reduces its ability to bind to the oocytes [2,3]. Therefore, improved techniques for long-term preservation of sheep’s semen are of great importance.
Researchers have been keen on using yolk as a cryoprotectant for preservation of animal semen. However, yolk is an animal-derived substance, and it might increase the pathogenic microorganisms during the process of transportation, producing substances that are not conducive to sperm survival. Also, there is no standardized operation that preserves the sperm. Researchers have been actively looking for new substances to replace yolk [4–6]. In addition, the yolk of the birds is more vulnerable to pathogens and might spread diseases during transportation in various countries. Therefore, the harm of using yolk as cryoprotectant should be avoided [7].
Recently, scientists are working on uncovering a new sperm protective agent that replaces yolk. It is necessary to effectively avoid the addition of animal-derived substances, and soybean lecithin (SL), which is a plant source, is currently being investigated [8]. It not only prevents the occurrence of health risks, but also improves the efficiency of semen preservation [9,10]. Recent studies revealed that SL assists in freezing bovine semen [11], ram [12,13], and goat [14].
SL is considered as a valuable source of plant-based phospholipids in commercial extenders that are used for freezing mammalian sperm without clearing the levels and adjustments due to trade protection. The Tris-fructose egg yolk dilution in our laboratory has a stable effect. The egg yolk in the dilution is replaced with soybean lecithin in equal amounts, and other components and factors remain unchanged. The content of lecithin in the yolk is about 10%, and the content of yolk in the diluent of Tris-fructose egg yolk is about 23%. The content of lecithin is converted to diluent by about 3%. The concentration of 1.25% to 10% SL was used instead of egg yolk to preserve the sheep sperm, and the effect of 1.25% concentration was considered much better, However, it was not determined whether adding soybean lecithin with concentration less than 1.25% had better effect [15]. Therefore, this study was designed to investigate the effect of 5 concentrations (0.25%, 0.5%, 0.75%, 1.0%, and 1.25%) of SL as an alternative for egg yolk in ram semen as an extender of sperm quality.
All animal experiments were conducted according to the Regulations and Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China, revised in 2004) and the study was approved by the Institutional Animal Care and Use Committee of Tarim University.
All experiments were performed in the Tarim University (40°54′ N, 81°30′ E) of Xinjiang of China. Semen samples obtained from six mature Duolang rams (2 to 3 years of age) were used in this study. The physical and sexual desire of these rams remained normal and they were capable of fertilizing and producing offspring through artificial insemination (AI). During the experiment, they were fed and managed uniformly. Rams were kept in a wide playground and had free access to water, conventionally. Carrots and eggs were fed 40 days before the experiment, and exercise was strengthened. From each ram, ejaculates were collected using artificial vagina ejaculation as described by Quinn et al [16]. The semen was collected from 18 ejaculates of six rams and was made into 3 test samples for each group.
After semen collection, the semen was transferred to the isothermal test tube immediately. The test tube was placed in insulation buckets at 37°C and sent back to the laboratory for evaluation in 30 min. Semen evaluation was carried out in accordance to the following criteria and was included in the study after meeting the criteria: ejaculation volume of 1 to 2 mL; sperm concentration of 1.5 to 2×109 sperm per mL; the percentage of progressive motile sperms should be higher than 80% and the percentage of abnormal sperm should be less than 10% for this experiment. Non-conforming semen samples were discarded. The semen samples were pooled to eliminate individual differences and divided into six equal aliquots and kept at 37°C in a water bath. After primary observation, the semen samples were diluted at a ratio of 1:4 (semen:diluent) at 37°C with different amounts of SL (0.25%, 0.5%, 0.75%, 1.0%, 1.25%) and control (23% egg yolk) in each group. The samples were placed in a refrigerator that was cooled to 0°C for 90 mins, and then ice cubes were added to the water bath for long-term storage of diluted semen for inspection.
The basic solution formula of Tris diluent: double distilled water (136 g), Tris (4.84 g), fructose (2.0 g), citric acid (2.68 g), adenosine triphosphate (100 mg), bovine serum albumin (300 mg), vitamin C (600 mg), penicillin (400,000 IU) and streptomycin (200,000 IU).
The sperm progressive motility was measured at 0, 24, 72, 144, 216, and 288 h after cooling. The semen was quickly and lightly mixed in the refrigerator, 10 μL semen dropped on the preheated slide, covered the slide, placed at 37°C hot table and detected the total number of sperm (≥200 sperm) in three visual fields at 400× under a microscope, and the number of sperms moving in a straight line were observed and counted at 37°C.
The sperm acrosome integrity was measured at 0, 24, 72, 144, 216, and 288 h after cooling. The sperm acrosome integrity was detected by a smear made from a drop of semen on a sterilized slide, followed by fixing with 95% ethanol for 30 minutes, staining with hematoxylin for 20 minutes, and then rinsing with distilled water. After staining with Eosin staining solution for 5 minutes, the purified water was washed and air-dried naturally. The stain was examined under a (400×) microscope. The total number of sperms in different fields (≥200 sperm) and the number of intact sperms are counted.
After the optimal SL concentration group was evaluated in the above experiment, the semen was collected again and stored in the control group and the optimal SL concentration group to prepare for AI. Duolang ewes (n = 223) housed in a free pasture were used for trans-cervical insemination. Ewes were synchronized with a Controlled Internal Drug Release for 14 days. Fifty-eight hours before AI, the sponges were removed, and animals were given an intramuscular injection of 500 to 600 IU of equine chorionic gonadotropin. Each ewe received two semen samples and saved till the ninth day of semen.
Ewes were randomly assigned to the control group (n = 112, ewes inseminated with control diluent) and the optimal SL concentration group (n = 111, ewes inseminated with the optimal SL diluent). Semen from the six rams were randomly injected to ewes and equally distributed for AI. Pregnancy was detected by B-mode ultrasonography on day 40. Pregnancy and lambing rate were recorded as an index of fertility and reproductive performance.
The sperm progressive motility rate, and acrosome integrity rate were presented as means±standard error of mean. The data were analyzed using analysis of variance and followed by Tukey’s HSD test to compare the differences between treatments (SPSS software; SPSS, Inc., Chicago, IL, USA). The pregnancy rate and the lambing rate were tested via Chi-square. The significance level of 5% was considered in all statistical analyses.
The sperm progressive motility showed no significant change with increasing SL dilution concentration at 0, 24, 72, and 144 h. However, after 216 h, the sperm progressive motility in the control group and 0.5% concentration groups was significantly higher when compared to 0.25% concentration (p<0.05), and the 0.5% concentration group had the best sperm progressive motility. There were no significant differences between the 0.5% concentration group and the control group. The progressive motility of these dilutions of ram sperm can still be higher than 50% at 216 h (Table 1).
At 0, 24, 72, 144, 216, and 288 h time periods, the sperm acrosome integrity rate of control group was not significantly different from that of the other groups (p>0.05), and the acrosome integrity rate of each group showed a downward trend with the prolongation of time (Table 2), but did not show significant changes with increasing dilution of SL (Figure 1).
The control group semen was preserved at 0°C. At 216 h, 112 ewes were artificially inseminated with control groups, in which 74 were pregnant, and the pregnancy rate was 66.1%. The 111 ewes artificially inseminated in 0.5% SL group showed that 72 were pregnant, with a pregnancy rate of 64.9%. Therefore, addition of SL and control in the dilution showed no significant effect on the pregnancy rate of sheep (p>0.05). There was no significant difference between the control group (63.4%) and the 0.5% concentration group (64.0%) on the lambing rate of sheep (Table 3).
The results showed that there was no difference in the sperm progressive motility, acrosome integrity, pregnancy rate and lambing rate between egg yolk dilution solution and when 0.5% SL was used to replace egg yolk for semen preservation. Therefore, 0.5% SL can replace egg yolk for long-term preservation of ram semen at 0°C.
More and more researchers have begun to study the cryopreservation of semen without animal-derived additives [17–19]. Our previous studies have shown that the concentration of 1.25% to 10% SL can be used instead of egg yolk to preserve the sheep sperm, and the effect of 1.25% concentration showed better results [15]. The current study revealed that 0.5% of SL can replace the egg yolk in the Tris dilution, so that the sheep sperm can be stored at 0°C for a longer time. It can be concluded from the literature that the optimal concentration range of SL for cryopreservation is 0.8% in dogs [20] to 1% in rams [14], humans [21] and cats [22] and goats [23]. The reason for this difference in addition of SL is that the differences in species are related to the differences of sperm plasma membrane composition between species [24]. The differences in the amount of concentration added might be due to different breeds of the rams, or different methods of preservation and different base liquor usage.
Our results showed that 0.5% concentration of SL dilution preserved sheep semen for 216 hours, had sperm progressive motility rate of 56.4%, the acrosome integrity rate of 75%, the pregnancy rate of 64.9%, and the lambing rate of 64.0%. Both were higher than the results revealed by Najafi et al [25] test, in which the sperm viability was 55.8% and membrane functionality was 44.5%, Mehdipour et al [26] test results showed that the viability of sperm was 41% and the membrane integrity was 49%, Forouzanfar et al [12] test results revealed that the sperm motility was 51.9%. The reason for these differences might be due to that when spermatozoa are preserved at low temperatures, cold blows must be prevented, which can in turn lead to irreversible sperm cells [27]. During the process of cryopreservation of spermatozoa, the process of freezing and thawing damages the ultrastructure of spermatozoa, leading to biochemical changes and loss of their vital content. The biochemical and ultrastructural changes of these spermatozoa under low temperatures might lead to a decrease of their functional integrity, survival rate and ability to bind to the ova [28]. The high concentration of SL has certain toxic effects on sperm motility and survival rate [12]. The addition of high concentration of SL in the diluent increased the viscosity of the diluent and particularly the debris in the diluent reduced the fertility of sperm together [29]. This might be subsequently related to the osmotic pressure of the diluent, decreasing with increasing concentration of SL in the diluent [30].
In conclusion, SL can successfully replace egg yolk as a supplement for preservation of sheep semen, without any adverse effects on sperm progressive motility, acrosome integrity, pregnancy rate, and lambing rate. Furthermore, our results showed that optimal lecithin concentration in the semen extender was 0.5% for preserving the ram semen. More investigations are warranted to accomplish evaluation of in vitro fertilizing ability of ram semen in the extender containing these concentrations of SL.
To our knowledge, this is the first report on the birth of lambs after the ewes were inseminated with ram semen stored for 216 hours. The ram sperm are capable of fertilization and producing normal offspring after preservation at 0°C with 0.5% of SL in Tris-based extender substituting for egg yolk.
ACKNOWLEDGMENTS
This study was financially supported by the Project of National Nature Science Foundation of China (Grant No. 31660657) and the Project of Innovation Team for efficient utilization of high-quality sheep resources around Tarim (Grant No. 2019CB010), This work was supported by Traim University (Xinjiang, China) and the Chinese Kashgar Animal Husbandry Workstation.
Figure 1
The sperm acrosome integrity image was taken at 144 h, in which the arrow marked sperm is acrosome intact. Sperm acrosome integrity rate were imaged at a magnification of 400× (Hematoxylin and eosin dyeing).
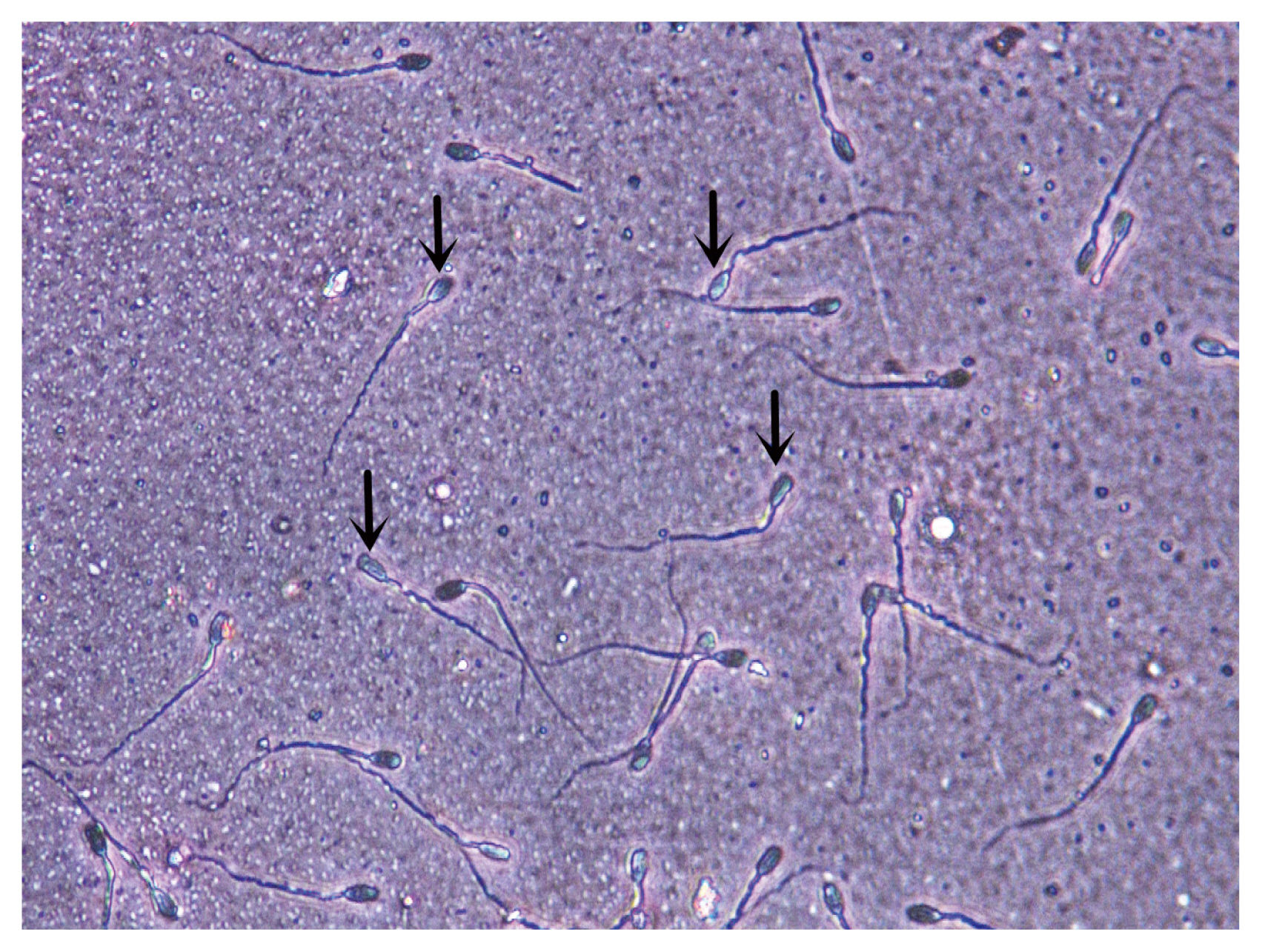
Table 1
Progressive motility of Duolang rams’ sperm stored at 0°C in diluents with different levels of soybean lecithin (mean±standard error of mean)
| Groups | 0 h | 24 h | 72 h | 144 h | 216 h | 288 h |
|---|---|---|---|---|---|---|
| Control | 91.9±4.9 | 86.7±4.2 | 76.4±6.4 | 66.1±3.2 | 56.2±3.2a | 49.3±3.7a |
| SL (0.25%) | 88.6±5.4 | 85.9±4.4 | 74.6±5.0 | 59.6±2.8 | 47.3±3.5b | 38.1±5.0b |
| SL (0.5%) | 88.4±3.7 | 84.9±3.4 | 74.8±2.9 | 66.4±3.3 | 56.4±2.6a | 48.3±2.9ab |
| SL (0.75%) | 90.5±2.6 | 84.7±3.9 | 72.2±1.3 | 65.1±1.4 | 54.6±3.0ab | 46.8±3.8ab |
| SL (1.0%) | 90.8±5.5 | 83.3±4.1 | 69.9±2.1 | 64.8±2.6 | 53.0±3.0ab | 46.6±2.7ab |
| SL (1.25%) | 89.2±5.6 | 83.4±4.6 | 75.7±4.6 | 64.3±3.1 | 53.3±2.7ab | 46.4±5.4ab |
Table 2
Acrosome integrity sperm of Duolang rams’ sperm in diluents supplemented with different levels of soybean lecithin (mean±standard error of mean)
REFERENCES
1. Benson JD, Woods EJ, Walters EM, Critser JK. The cryobiology of spermatozoa. Theriogenology 2012; 78:1682–99.
https://doi.org/10.1016/j.theriogenology.2012.06.007


2. Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology 2004; 48:146–56.
https://doi.org/10.1016/j.cryobiol.2004.03.002


3. Barbas JP, Mascarenhas RD. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 2009; 10:49–62.
https://doi.org/10.1007/s10561-008-9081-4



4. Papa FO, Felicio GB, Melo-Ona CM, et al. Replacing egg yolk with soybean lecithin in the cryopreservation of stallion semen. Anim Reprod Sci 2011; 129:73–7.
https://doi.org/10.1016/j.anireprosci.2011.10.006


5. Akhter S, Ansari MS, Andrabi SMH, Ullah N, Qayyum M. Effect of antibiotics in extender on bacterial and spermatozoal quality of cooled buffalo (Bubalus bubalis) bull semen. Reprod Domest Anim 2008; 43:272–8.
https://doi.org/10.1111/j.1439-0531.2007.00890.x


6. Althouse GC. Sanitary procedures for the production of extended semen. Reprod Domest Anim 2008; 43:374–8.
https://doi.org/10.1111/j.1439-0531.2008.01187.x


7. Holt WV. Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology 2000; 53:47–58.
https://doi.org/10.1016/S0093-691X(99)00239-3


8. Chelucci S, Pasciu V, Succu S, et al. Soybean lecithin-based extender preserves spermatozoa membrane integrity and fertilizing potential during goat semen cryopreservation. Theriogenology 2015; 83:1064–74.
https://doi.org/10.1016/j.theriogenology.2014.12.012


9. Andrabi SMH. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim 2009; 44:552–69.
https://doi.org/10.1111/j.1439-0531.2008.01240.x


10. Akhter S, Ansari MS, Andrabi SMH, Rakha BA, Ullah N, Khalid M. Soya-lecithin in extender improves the freezability and fertility of buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim 2012; 47:815–9.
https://doi.org/10.1111/j.1439-0531.2011.01973.x


11. Aires VA, Hinsch KD, Mueller-Schloesser F, Bonger K, Mueller-Schloesser S, Hinsch E.
In vitro and in vivo comparison of egg yolk-based and soybean lecithin-based extenders for cryopreservation of bovine semen. Theriogenology 2003; 60:269–79.
https://doi.org/10.1016/S0093-691X(02)01369-9


12. Forouzanfar M, Sharafi M, Hosseini SM, et al.
In vitro comparison of egg yolk-based and soybean lecithin-based extenders for cryopreservation of ram semen. Theriogenology 2010; 73:480–7.
https://doi.org/10.1016/j.theriogenology.2009.10.005


13. Roof DJ, Bowley S, Price LL, Matsas DJ. Comparison of two commercial extenders for cryopreservation of goat semen without sperm washing. Theriogenology 2012; 77:412–20.
https://doi.org/10.1016/j.theriogenology.2011.08.015


14. Salmani H, Towhidi A, Zhandi M, Bahreini M, Sharafi M.
In vitro assessment of soybean lecithin and egg yolk based diluents for cryopreservation of goat semen. Cryobiology 2014; 68:276–80.
https://doi.org/10.1016/j.cryobiol.2014.02.008


15. Zhao J, Wang J, Li N, Gao Q. Effect of containing soybean lecithin instead of yolk dilution for low temperature preservation of sheep semen. J Tarim Univ 2018; 30:15–9.
16. Quinn PJ, Salamon S, White IG. The effect of cold shock and deep-freezing on ram spermatozoa collected by electrical ejaculation and by an artificial vagina. Aust J Agric Res 1968; 19:119–28.
https://doi.org/10.1071/AR9680119

17. de Paz P, Esteso MC, Alvarez M, Mata M, Chamorro CA, Anel L. Development of extender based on soybean lecithin for its application in liquid ram semen. Theriogenology 2010; 74:663–71.
https://doi.org/10.1016/j.theriogenology.2010.03.022


18. Toker MB, Alcay S, Gokce E, Ustuner B. Cryopreservation of ram semen with antioxidant supplemented soybean lecithin-based extenders and impacts on incubation resilience. Cryobiology 2016; 72:205–9.
https://doi.org/10.1016/j.cryobiol.2016.05.001


19. Masoudi R, Sharafi M, Shahneh AZ, et al. Fertility and flow cytometry study of frozen-thawed sperm in cryopreservation medium supplemented with soybean lecithin. Cryobiology 2016; 73:69–72.
https://doi.org/10.1016/j.cryobiol.2016.05.010


20. Kmenta I, Strohmayer C, Muller-Schlosser F, Schafer-Somi S. Effects of a lecithin and catalase containing semen extender and a second dilution with different enhancing buffers on the quality of cold-stored canine spermatozoa. Theriogenology 2011; 75:1095–103.
https://doi.org/10.1016/j.theriogenology.2010.11.018


21. Reed ML, Ezeh PC, Hamic A, Thompson DJ, Caperton CL. Soy lecithin replaces egg yolk for cryopreservation of human sperm without adversely affecting postthaw motility, morphology, sperm DNA integrity, or sperm binding to hyaluronate. Fertil Steril 2009; 92:1787–90.
https://doi.org/10.1016/j.fertnstert.2009.05.026


22. Vick MM, Bateman HL, Swanson WF. 97 Improved cryopreservation of domestic cat spermatozoa in a soy lecithin-based extender. Reprod Fertil Dev 2010; 23:153–4.
https://doi.org/10.1071/RDv23n1Ab97

23. Roof DJ, Bowley S, Price LL, Matsas DJ. Comparison of two commercial extenders for cryopreservation of goat semen without sperm washing. Theriogenology 2012; 77:412–20.
https://doi.org/10.1016/j.theriogenology.2011.08.015


24. Bencharif D, Amirat L, Anton M, et al. The advantages of LDL (low density lipoproteins) in the cryopreservation of canine semen. Theriogenology 2008; 70:1478–88.
https://doi.org/10.1016/j.theriogenology.2008.06.095


25. Najafi A, Kia HD, Mohammadi H, et al. Different concentrations of cysteamine and ergothioneine improve microscopic and oxidative parameters in ram semen frozen with a soybean lecithin extender. Cryobiology 2014; 69:68–73.
https://doi.org/10.1016/j.cryobiol.2014.05.004


26. Mehdipour M, Kia HD, Najafi A, Dodaran HV, García-Álvarez O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology 2016; 73:297–303.
https://doi.org/10.1016/j.cryobiol.2016.10.008


27. Salamon S, Maxwell WMC. Storage of ram semen. Anim Reprod Sci 2000; 62:77–111.
https://doi.org/10.1016/S0378-4320(00)00155-X


28. Salamon S, Maxwell WMC. Frozen storage of ram semen II. Causes of low fertility after cervical insemination and methods of improvement. Anim Reprod Sci 1995; 38:1–36.
https://doi.org/10.1016/0378-4320(94)01328-J

29. van Wagtendonk-de Leeuw AM, Haring RM, Kaal-Lansbergen LMTE, den Daas JHG. Fertility results using bovine semen cryopreserved with extenders based on egg yolk and soy bean extract. Theriogenology 2000; 54:57–67.
https://doi.org/10.1016/S0093-691X(00)00324-1


30. Salmani H, Towhidi A, Zhandi M, Bahreini M, Sharafi M.
In vitro assessment of soybean lecithin and egg yolk based diluents for cryopreservation of goat semen. Cryobiology 2014; 68:276–80.
https://doi.org/10.1016/j.cryobiol.2014.02.008


- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





