 |
 |
| Anim Biosci > Volume 32(9); 2019 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
ACKNOWLEDGMENTS
Figure 1

Figure 2
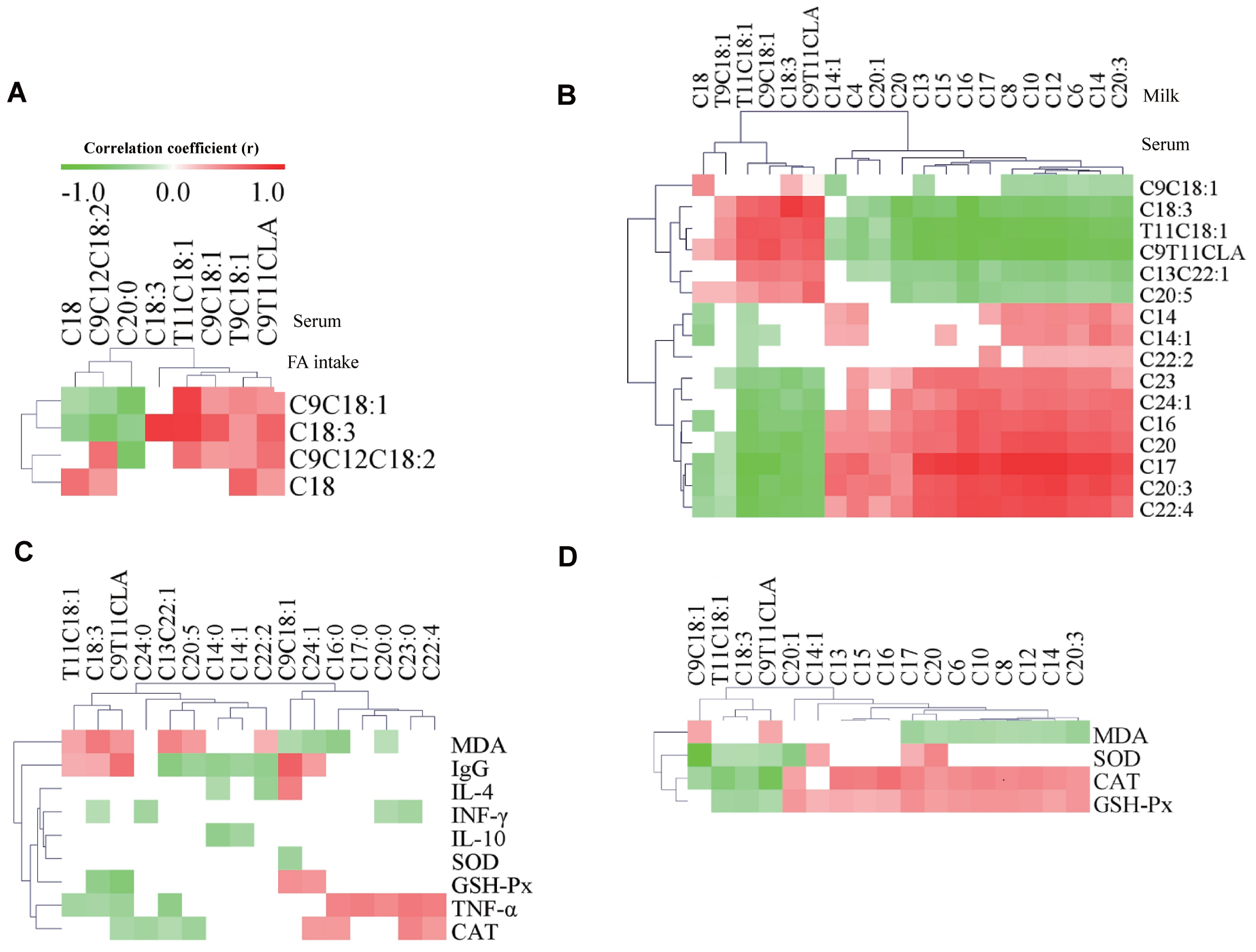
Table 1
| Items | Treatment1) | SEM | p-value2) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CON | RO | FO | RFO | CON vs oil | RO vs FO | RFO vs RO+FO | ||
| DMI (kg/d) | 20.2 | 20.5 | 19.8 | 19.9 | 0.26 | 0.45 | 0.67 | 0.37 |
| Energy intake (Mcal/d) | 32.5 | 35.5 | 34.3 | 34.4 | 0.81 | 0.39 | 0.68 | 0.36 |
| Fatty acid intake (g/d) | ||||||||
| C14:0 | 2.3 | 3.0 | 2.5 | 2.5 | 0.22 | *** | * | 0.45 |
| C16:0 | 105.3 | 161.5 | 127.6 | 140.7 | 1.85 | *** | ** | 0.54 |
| C16:1 | 3.2 | 4.7 | 3.1 | 3.9 | 0.68 | ** | ** | 0.37 |
| C17:0 | 1.0 | 1.3 | 1.3 | 1.3 | 0.28 | ** | 0.88 | 0.87 |
| C18:0 | 15.0 | 68.5 | 37.0 | 50.6 | 2.15 | *** | ** | 0.55 |
| C18:1 cis-9 | 127.4 | 284.3 | 246.1 | 260.4 | 4.86 | *** | * | 0.44 |
| C18:2 cis-9,12 | 259.0 | 526.4 | 344.4 | 423.9 | 5.72 | *** | *** | 0.55 |
| C18:3 (ALA) | 52.6 | 240.1 | 490.1 | 370.6 | 4.89 | *** | ** | 0.77 |
| C20:0 | 3.0 | 5.1 | 3.4 | 4.1 | 0.19 | ** | ** | 0.43 |
| C20:1 | 1.3 | 2.8 | 2.5 | 2.5 | 0.11 | *** | 0.76 | 0.45 |
| C22:0 | 2.6 | 3.3 | 3.3 | 3.3 | 0.18 | * | 0.87 | 0.83 |
| C22:2 | 2.7 | 2.9 | 2.8 | 2.7 | 0.11 | * | 0.44 | 0.34 |
| Summations (g/d) | ||||||||
| Total C183) | 454.0 | 1,119.3 | 1,117.6 | 1,105.4 | 9.28 | *** | 0.58 | 0.46 |
| SFA | 132.4 | 248.1 | 180.2 | 207.4 | 4.76 | ** | ** | 0.65 |
| UFA | 446.2 | 1,061.1 | 1,089.0 | 1,064.0 | 8.57 | *** | 0.76 | 0.29 |
| MUFA | 132.0 | 291.7 | 251.7 | 266.8 | 4.75 | *** | * | 0.77 |
| PUFA | 314.3 | 769.4 | 837.3 | 797.2 | 6.74 | *** | ** | 0.65 |
SEM, standard error of least squares means; DMI, dry matter intake; ALA, α-linolenic acid; SFA, saturated fatty acid; UFA, unsaturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
1) Cows were fed a basal diet (control; CON) or basal diet supplemented with either 4.0% rubber seed oil (RO), 4.0% flaxseed oil (FO), or 2.0% rubber seed oil+2.0% flaxseed oil (RFO). The CON diet was also used for feeding during the pre-trial period.
2) CON vs oil, CON versus oil (RO, FO, RFO); RO vs FO, RO versus FO; RFO vs RO+FO, RFO versus RO plus FO.
Table 2
| FA (g/100 g of total FA reported) | Treatment1) | SEM | p-value2) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CON | RO | FO | RFO | CON vs oil | RO vs FO | RFO vs RO+FO | ||
| C8:0 | 0.23 | 0.28 | 0.24 | 0.33 | 0.02 | ** | 0.09 | ** |
| C14:0 | 0.98 | 0.76 | 0.89 | 0.87 | 0.05 | ** | * | 0.35 |
| C14:1 | 0.49 | 0.38 | 0.46 | 0.46 | 0.02 | * | * | 0.14 |
| C15:0 | 0.54 | 0.37 | 0.43 | 0.41 | 0.02 | *** | ** | 0.37 |
| C16:0 | 8.75 | 7.81 | 7.60 | 7.59 | 0.16 | *** | 0.31 | 0.52 |
| C16:1 | 0.52 | 0.67 | 0.69 | 0.71 | 0.04 | *** | 0.61 | 0.55 |
| C17:0 | 0.72 | 0.44 | 0.47 | 0.44 | 0.02 | *** | 0.21 | 0.44 |
| C18:0 | 12.99 | 12.79 | 12.18 | 11.75 | 0.28 | * | 0.11 | * |
| C18:1 t-93) | 0.16 | 0.31 | 0.28 | 0.41 | 0.05 | ** | 0.70 | 0.06 |
| C18:1 t-11 (VA) | 0.41 | 2.16 | 2.70 | 2.89 | 0.19 | *** | * | * |
| C18:1 c-9 | 9.35 | 11.28 | 10.45 | 10.37 | 0.56 | * | 0.26 | 0.45 |
| C18:2 c-9, 12 | 48.81 | 47.50 | 43.97 | 45.54 | 0.81 | *** | ** | 0.83 |
| C20:0 | 1.38 | 0.70 | 0.59 | 0.73 | 0.08 | *** | 0.32 | 0.33 |
| C18:3 (ALA) | 4.42 | 6.84 | 10.74 | 8.37 | 0.25 | *** | *** | 0.17 |
| C20:1 | ND | 0.03 | 0.03 | ND | 0.00 | *** | * | *** |
| C18:2 c-9, t-11 CLA | 0.01 | 0.32 | 0.43 | 0.46 | 0.03 | *** | * | * |
| C22:0 | 0.22 | 0.22 | 0.26 | 0.23 | 0.01 | * | *** | 0.10 |
| C20:3 c-8, 11, 14 | 3.58 | 1.37 | 1.61 | 1.58 | 0.16 | *** | 0.27 | 0.64 |
| C23:0 | 2.91 | 2.35 | 2.17 | 2.43 | 0.13 | *** | 0.30 | 0.24 |
| C22:1 c-13 | 0.47 | 052 | 0.63 | 0.59 | 0.02 | *** | ** | 0.51 |
| C20:5 (EPA) | 0.62 | 0.74 | 0.90 | 0.90 | 0.05 | *** | * | 0.16 |
| C22:2 | 0.27 | 0.23 | 0.28 | 0.25 | 0.01 | * | *** | 0.69 |
| C24:1 | 0.21 | 0.16 | 0.16 | 0.16 | 0.01 | *** | 0.65 | 0.84 |
| C22:4 | 0.87 | 0.39 | 0.40 | 0.40 | 0.06 | *** | 0.89 | 0.99 |
| C22:5 | 0.90 | 0.99 | 0.99 | 1.09 | 0.07 | 0.12 | 0.99 | 0.26 |
| Summations | ||||||||
| SFA4) | 28.80 | 25.50 | 25.20 | 25.21 | 0.48 | *** | 0.93 | 0.45 |
| UFA | 71.20 | 74.43 | 74.80 | 74.75 | 0.48 | *** | 0.91 | 0.34 |
| MUFA | 11.72 | 15.50 | 15.40 | 15.96 | 0.58 | *** | 0.76 | 0.44 |
| PUFA | 59.48 | 59.61 | 59.40 | 58.77 | 0.75 | 0.78 | 0.96 | 0.19 |
| (n-3)/(n-6) ratio | 0.21 | 0.22 | 0.33 | 0.27 | 0.01 | *** | *** | 0.55 |
FA, fatty acid; SEM, standard error of least squares means; VA, vaccenic acid; ALA, α-linolenic acid; ND, not detected; CLA, conjugated linoleic acid; EPA, eicosapentaenoic acid; SFA, saturated fatty acid; UFA, unsaturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
1) Cows were fed a basal diet (control; CON) or basal diet supplemented with either 4.0% rubber seed oil (RO), 4.0% flaxseed oil (FO), or 2.0% rubber seed oil+2.0% flaxseed oil (RFO). The CON diet was also used for feeding during the pre-trial period.
2) CON vs oil, CON versus oil (RO, FO, RFO); RO vs FO, RO versus FO; RFO vs RO+FO, RFO versus RO plus FO.
4) SFA, sum of C8:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C22:0, and C23:0; UFA, total unsaturated FA reported; MUFA, sum of C14:1, C16:1, C18:1 trans-9, C18:1 trans-11 (VA), C18:1 cis-9, C20:1, C22:1 cis-13, and C24:1; PUFA, sum of C18:2 cis-9, 12, C18:2 cis-9, trans-11 (CLA), C18:3 (ALA), C20:3 cis-8, 11, 14, C20:5 (EPA), C22:2, C22:4, and C22:5.
Table 3
| Items | Treatment1) | SEM | p-value2) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CON | RO | FO | RFO | CON vs oil | RO vs FO | RFO vs RO+FO | ||
| Serum | ||||||||
| SOD (U/mL) | 42.7 | 41.8 | 41.7 | 47.6 | 2.68 | 0.74 | 0.99 | 0.10 |
| GSH-Px (U/mL) | 122.0 | 118.7 | 102.3 | 104.4 | 4.23 | ** | * | 0.24 |
| MDA (nmol/mL) | 3.8 | 3.3 | 4.8 | 4.6 | 0.27 | 0.10 | ** | 0.19 |
| CAT (U/mL) | 2.5 | 2.3 | 2.0 | 1.8 | 0.17 | * | 0.37 | 0.27 |
| Milk | ||||||||
| SOD (U/mL) | 133.2 | 127.9 | 130.6 | 130.4 | 1.44 | * | 0.23 | 0.56 |
| GSH-Px (U/mL) | 72.7 | 58.8 | 69.9 | 56.8 | 5.28 | 0.09 | 0.19 | 0.28 |
| MDA (nmol/mL) | 1.9 | 3.5 | 2.1 | 2.6 | 0.40 | * | 0.48 | 0.12 |
| CAT (U/mL) | 5.3 | 4.0 | 3.5 | 3.0 | 0.31 | ** | 0.46 | 0.23 |
SEM, standard error of least squares means; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; CAT, catalase.
1) Cows were fed a basal diet (control; CON) or basal diet supplemented with either 4.0% rubber seed oil (RO), 4.0% flaxseed oil (FO), or 2.0% rubber seed oil+2.0% flaxseed oil (RFO). The CON diet was also used for feeding during the pre-trial period.
Table 4
| Items | Treatment1) | SEM2) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CON | RO | FO | RFO | CON vs oil | RO vs FO | RFO vs RO+FO | ||
| IgA (μg/mL) | 58.1 | 73.5 | 67.4 | 58.6 | 1.86 | 0.44 | 0.58 | 0.19 |
| IgG (mg/mL) | 30.5 | 42.5 | 24.6 | 23.9 | 3.15 | * | *** | 0.97 |
| IgM (mg/mL) | 4.6 | 3.9 | 3.6 | 4.3 | 0.63 | 0.42 | 0.73 | 0.50 |
| PGE2 (pg/mL) | 12.6 | 14.6 | 12.1 | 12.1 | 0.55 | 0.10 | ** | 0.71 |
| Cytokine levels (pg/mL) | ||||||||
| IFN-γ | 230.1 | 213.3 | 215.2 | 216.9 | 1.91 | *** | 0.38 | 0.42 |
| IL-2 | 45.3 | 50.9 | 45.3 | 47.9 | 1.85 | 0.37 | 0.29 | 0.59 |
| IL-4 | 21.4 | 22.4 | 21.4 | 21.4 | 0.50 | 0.36 | * | 0.08 |
| IL-10 | 204.1 | 209.2 | 201.4 | 199.9 | 1.52 | 0.58 | 0.07 | 0.22 |
| TNF-α | 67.6 | 16.3 | 26.0 | 23.9 | 5.79 | ** | 0.89 | 0.58 |
SEM, standard error of least squares means; Ig, immunoglobulin; PGE2, prostaglandin 2; INF-γ, interferon γ; IL, interleukins; TNF-α, tumor necrosis factor α.
1) Cows were fed a basal diet (control; CON) or basal diet supplemented with either 4.0% rubber seed oil (RO), 4.0% flaxseed oil (FO), or 2.0% rubber seed oil+2.0% flaxseed oil (RFO). The CON diet was also used for feeding during the pre-trial period.
REFERENCES
- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print





