 |
 |
| Anim Biosci > Volume 31(2); 2018 > Article |
|
Abstract
Objective
Examine the effects of supplementing bahiagrass hay (BG) with potentially anthelmintic quantities of hays of perennial peanut (PEA) or sericea lespedeza (LES) or seeds of velvet bean (Mucuna pruriens L.; MUC) or papaya (PAP) on the intake and nutritive value (Experiment 1), and the performance and parasite burden (Experiment 2) of goats.
Methods
In Experiment 1, 38 male goats (27.4┬▒5.7 kg body weight) were randomly assigned to each of 5 treatments: i) BG alone and BG plus; ii) PEA; iii) LES; iv) MUC; and v) PAP. Goats were fed for ad libitum consumption and adapted to the diets for 14 d followed by 7 d of measurement. The PEA, LES, MUC (50%, 50%, and 10% of the diet dry matter [DM], respectively), and PAP (forced-fed at 10 g/d) were fed at rates that would elicit anthelmintic effects. In Experiment 2, goats remained in the same treatments but were allocated to 15 pens (3 pens per treatment) from d 22 to 63. All goats were infected with parasites by grazing an infected bahiagrass pasture from 0800 to 1500 h daily and then returned to the pens.
Results
Dry matter intake tended to be greater in goats fed PEA and LES than those fed BG (757 and 745 vs 612 g/d, respectively). Digestibility of DM (59.5% vs 54.9%) and organic matter (60.8% vs 56.0%) were greater in goats fed MUC vs BG, respectively. In Experiment 2, feeding PAP, LES, and PEA to goats reduced nematode fecal egg counts by 72%, 52%, and 32%, reduced abomasal adult worm counts by 78%, 52%, and 42%, and decreased plasma haptoglobin concentrations by 42%, 40%, and 45% relative to feeding BG alone, respectively.
The US goat industry has become an important livestock enterprise because of the high ethnic minority demand for chevon [1]. However, successful goat production requires proper control of gastrointestinal nematodes (GIN) and coccidian species, such as Haemonchus contortus (H. contortus) and Eimeria spp., respectively. H. contortus, is the main cause of ill health and low productivity in grazing goats in tropical and subtropical regions [2]. Haemonchosis is characterized by anemia, extreme weakness, loss of condition, and eventually death [3]. On the other hand, coccidian subclinical infections can also cause production losses for the small ruminant producer due to enteric disease that results in diarrhea, low weight gains, and occasionally death [4]. Economic losses arising from decreased production due to the infection, the costs of prophylaxis and treatment, and the death of infected animals amount to millions of dollars per year in the US [5].
The effectiveness of anthelmintic treatments is a major determinant of the productivity of goats. Yet, nematode parasites of goats have become more resistant to anthelmintic drugs in recent years and have become a major concern to small ruminant producers in the Southeast of the United States and worldwide [6]. Consequently, natural feed supplements have been investigated as alternative treatments and control strategies. For instance, tannin-rich sericea lespedeza (Lespedeza cuneate [Dum-Cuors] G. Don; LES) reduced GIN parasite burdens in goats [7] and velvet bean (Mucuna pruriens L. [M. pruriens L.]; MUC) paralyzed intestinal worms in lambs [8]. Papaya (Carica papaya L.; PAP) also inhibited the growth of H. contortus in ewes [9] because of the benzyl isothiocyanate it contains. Boosting the immune system of goats by feeding protein-rich legumes such as perennial peanut (Arachis glabrata Benth.; PEA) or LES may also aid in reducing parasite burdens in goats. However, only a few studies have simultaneously compared nutritional antiparasitic approaches to those dependent on bioactive secondary compounds in plants in goats. Therefore, the objective of this study was to investigate the effects of supplementing bahiagrass hay (Paspalum notatum Fl├╝gge; BG) with potentially anthelmintic quantities of hays of PEA or LES or seeds of MUC or PAP on the feed intake, digestibility, N balance, ruminal fermentation, performance, parasite burden, and health of goats.
A 6-wk regrowth of BG (cv. Pensacola) and a primary growth of PEA (cv. Florigraze) were harvested from established stands at the University of Florida Beef Research Unit, Gainesville, FL, USA and stored in a hay barn as 440-kg round hay bales. The LES hay (cv. AU Grazer) and MUC (cv. Aterrima) and PAP (cv. Criolla) seeds were purchased from producers (New Bern, NC, USA; Orlando, FL, USA; and Lima, Peru, respectively).
The experiment was conducted at the University of Florida Sheep Unit (Gainesville, FL, USA) and it lasted for 120 d (December 2011 to April 2012). In Experiment 1, goats were housed in metabolism cages (100├Ś40├Ś80 cm) adapted for urine collection inside an open-sided barn. In Experiment 2, goats were housed in an open-sided barn with 15 pens of 20 m2 each fitted with concrete floors and automatic water troughs. Access to a bahiagrass pasture was available next to the barn. During both experiments, the mean temperature and relative humidity were 15.5┬░C and 78.7%, with minima of 8.4┬░C and 38% and maxima of 23.5┬░C and 98% [10].
All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee. In Experiment 1, 38 Boer├ŚSpanish├ŚKiko castrated male goats (27.4┬▒5.7 kg body weight [BW]) were treated for coccidiosis with amprolium (Corid, Merial, GA, USA; 5 mg/kg BW) and dewormed with albendazole (Valbazen, Zoetis, NJ, USA; 10 mg/kg BW) and moxidectin (Cydectin, Boeringer Ingelheim, Ingelheim, Germany; 0.4 mg/kg BW). After confirming all goats had low GIN fecal egg counts (FEC, <50 eggs/g) and coccidian fecal oocyst counts (FOC, <50 eggs/g), goats were weighed for 2 consecutive days, stratified by BW and randomly assigned to each of 5 treatments: i) BG hay alone; ii) BG hay plus PEA hay; iii) BG hay plus LES hay; iv) BG hay plus MUC seed; and v) BG hay plus PAP seed. All treatments had 8 replicate goats except PAP, which had 6 goats. Goats were fitted with canvas feces collection bags and fed for ad libitum consumption (110% of the previous day intake) in 2 equal allotments at 0800 and 1600 h and animals had access to water at all times. Goats were adapted to the diets for 14 d before the 7 d measurement period. The treatments were fed at levels that reflected their potential to reduce the parasite burden via direct anthelmintic effects of bioactive components, indirect anthelmintic effects due to improved nutrient supply and hence improved immunity or a combination of both factors. The PEA and LES hays were 50% of the diet dry matter [DM], MUC was 10% of the diet DM, and PAP was provided at 10 g/d. The MUC, PEA, and LES were also fed at levels that would supply similar amounts of supplemental protein. The inclusion level of PAP was based on recommended levels (Carcelen, F. and Camacho, J. Personal communication) for controlling GIN without adversely affecting animal health. PAP was orally dosed by gavage to ensure complete consumption but the other supplements were fed. Also, 18 g/head/d of a mineral mix was thoroughly mixed with the concentrate before feeding (United Salt Corp., Ranch House Trace Mineralized Salt, Houston, TX, USA). The mineral mix contained 88% NaCl, 2.5% Ca, 1% S, 1,500 mg/kg Fe, 3,000 mg/kg Mn, 2,500 mg/kg Zn, 25 mg/kg Co, 150 mg/kg Cu, 90 mg/kg, and 10 mg/kg Se.
Experiment 2 was designed to measure treatment effects on the growth, parasite burden and health of goats. It was started on d 22 and it lasted for 63 d. Goats continued under the same treatments as in Experiment 1 but were allocated to a total of 15 pens that consisted of 3 pens per treatment (2 to 3 goats per pen). All goats were naturally infected with parasites by allowing them to graze an adjacent bahiagrass pasture infected with L-3 stage larvae of GIN and coccidia infective oocysts from 0800 to 1500 h daily and then returned to the pens where the same diets as in Experiment 1 were offered for ad libitum consumption (110% of the previous day intake). The dietary treatments were hand-mixed and offered in the same feeder at 0800 and 1600 and supplemented with a mixture of corn (Zea mays) and soybean meal (Glycine max) containing 15.4% crude protein (CP) and 84.5% total digestible nutrients at a rate of 150 g/head/d. Water was provided ad libitum and 18 g/head/d of a mineral premix (United Salt Corp., Ranch House Trace Mineralized Salt, Houston, TX, USA) was fed.
In Experiment 1, samples of each feed were taken daily during the 7-d measurement period and daily refusals were weighed and stored. Total fecal and urine output were collected daily from each goat, weighed, and a 10% subsample was stored (ŌłÆ20┬░C) for further analysis. Goats were weighed and blood sampled by jugular venipuncture on day 0 and 21. A Vacutainer tube (BD, Franklin Lakes, NJ, USA) containing sodium heparin anticoagulant was used to collect approximately 20 mL of whole blood from each goat and the tubes were stored on ice. The blood was centrifuged at 1,920├Śg for 20 min at 4┬░C to separate the plasma, which was decanted and stored at ŌłÆ20┬░C until analyzed. Ruminal fluid was collected from 30 randomly selected goats (6 per treatment) on d 21 by aspiration from orally-inserted stomach tubes 3 h after the morning feeding. A representative (100 mL) sample was analyzed immediately for pH (Accumet, model XL 25, Fischer Scientific, Pittsburg, PA, USA) and acidified with 9.0 M H2SO4 to pH 2, centrifuged for 30 min at 4┬░C and 2,795├Śg, and frozen (ŌłÆ20┬░C) for subsequent analysis.
Samples of feed were dried at 60┬░C for 48 hours in a forced air oven and ground to pass through a 1-mm screen in a Wiley mill (Arthur H. Thomas Company, Philadelphia, PA, USA). Samples were analyzed for ash by combustion in a muffle furnace at 600┬░C overnight [11]. Total N concentration was determined by the Dumas combustion method [11] using a Vario MAX CN Macro Elementar Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) and used to calculate CP concentration (CP = total N├Ś 6.25). The neutral detergent fiber (NDF) and acid detergent fiber (ADF) concentrations [12] were determined using an ANKOM 200 Fiber Analyzer (Ankom, Macedon, NY, USA). Samples were also analyzed for acid detergent lignin (ADL) according to Van Soest et al [12]. Amylase was used for NDF analysis but sodium sulfite was not and the results were expressed inclusive of residual ash. The 3,4-dihydroxy-L-phenylalanine (L-dopa) in MUC was analyzed by high-performance liquid chromatography (HPLC) with UV detection using the method of Siddhuraju and Becker [13]. Phenolic compounds in LES were extracted as described by Terrill et al [14] and proanthocyanidins were quantified as described by Buran et al [15] with an Agilent 1200 HPLC system consisting of a binary pump, an autosampler, and a fluorescence detector (Agilent Technologies, Palo Alto, CA, USA). A Phenomenex Luna 5u silica column (250├Ś4.60 mm, 5 ╬╝m) with a silica pre-column security guard cartridge was used for the separation of proanthocyanidins. The binary mobile phase consisted of (A) methylene chloride:methanol:water:acetic acid (82:14:2:2, v/v/v/v) and (B) methanol:water:acetic acid (96:2:2, v/v/v). The column temperature was set at 37┬░C. Epicatechin was used as the external standard to quantify proanthocyanidins. The quantity of benzyl isothiocyanate in PAP was analyzed via gas chromatography coupled with electron impact (EI) ŌĆō mass spectroscopy. Briefly, a 0.5 ╬╝L portion of a methanol solution of the PAP extract was injected into a Trace GC Ultra gas chromatograph (Thermo Scientific, Waltham, MA, USA) equipped with a 30-m Stabilwax-DA column (Restek Corporation, Bellefonte, PA, USA). The column temperature was held at 50┬░C for 1 min, and then heated at 30┬░ per min to 260┬░C. The eluent underwent electron impact ionization and the ions were detected with a DSQ quadrupole mass spectrometer (Thermo Scientific Electron Corporation, Madison, WI, USA).
The volatile fatty acids (VFA) in ruminal fluid were measured using the method of Muck and Dickerson [16] and a HPLC system (Hitachi LaChrom Elite, Tokyo, Japan) coupled to a UV detector (Hitachi L-2400, Japan) set at 210 nm. The column was a Bio-Rad Aminex HPX-87H (Bio-Rad laboratories, Hercules, CA, USA) with 0.015 M H2SO4 mobile phase and a flow rate of 0.7 mL/min at 45┬░C. Ruminal fluid ammonia-N concentration was determined by an ALPKEM auto analyzer (ALKPEM Corporation, Clackamas, OR, USA) and an adaptation of the Noel and Hambleton [17] procedure that involved colorimetric quantification of N. Plasma glucose and blood urea nitrogen (BUN) concentration were measured using adaptations for a Technicon Autoanalyzer II (Bran-Luebbe, Elinsford, NY, USA) of methods of Gochman and Schmitz [18], and Coulombe and Favreau [19].
In Experiment 2, samples of each feed, jugular blood, and feces were taken weekly during the 63-d experiment and stored for further analysis. Approximately 3 mL of blood plasma were collected, processed, and stored as described in Experiment 1. Fecal samples (approx. 4 g) were collected directly from the rectum, transported on ice, and immediately analyzed for FEC and FOC with the modified McMaster procedure [20]. Goat BW was measured for 2 consecutive days at the beginning and end of the 9-wk experiment and subsequently averaged; interim full BW was recorded weekly. Plasma haptoglobin concentrations were determined by measuring haptoglobin/hemoglobin complexing based on differences in peroxidase activity [21]. Goats were monitored for evidence of haematophagous worm burden using the FAMACHA eye chart [22]. On d 63, goats were slaughtered at a USDA approved abattoir and adult worms in their abomasums were counted using a dissecting microscope [23].
A completely randomized design with 8 replicate goats per treatment (except PAP, n = 6) was used to determine effects of the 5 treatments on the feed intake, digestibility, N balance, and ruminal fermentation in Experiment 1. The model for analyzing the animal measurements included the effect of treatment and goat (random effect). For the forage nutritional composition data, the model included the effect of treatment and the replicates were the 7 daily samples collected during the experimental period. Data were analyzed with the MIXED procedure of SAS v 9.3 (SAS Inst., Inc., Cary, NC, USA) and FisherŌĆÖs F-protected Least Significance Difference test was used for mean separation.
A randomized complete block design with 3 experimental units (pens) per treatment was used to determine effects of the 5 treatments on the performance, parasite burden, and health of goats in Experiment 2. The model for analyzing animal measurements included treatment, pen (random term), time (repeated measure) and treatment├Śtime effects. Data were analyzed with the MIXED procedure of SAS v 9.3 (SAS Inst., Inc., Cary, NC, USA) and FisherŌĆÖs F-protected Least Significance Difference test was used for mean separation. The covariance structure with the least Akaike information criterion was chosen for each repeated measure analysis performed. The slice command was used to detect differences between treatments at specific time points. For all data, the distribution of residuals was examined for normality using the normal probability, quantile-quantile, and predicted mean plots options of SAS. The FEC and FOC data were log transformed. Significance was declared at p<0.05, and only significant interaction effects were discussed. Tendencies were declared at p>0.05 and Ōēż0.10.
The experiment was conducted from January to April, when GIN parasite infection levels are less than those in the summer and fall [24].
Nutritional composition of diets: Among the hays, PEA had slightly lower (p<0.01) DM and organic matter (OM) concentrations than BG and LES but CP concentrations were greater in the legumes (Table 1). In contrast, NDF concentration was greater in BG (p<0.01), followed by LES and PEA. Concentration of ADF was greater in LES (p<0.01), followed by PEA and BG. The metabolizable energy (ME) was calculated based on feed composition [25, 26] and was greater in PEA (p<0.01), followed by LES and BG (p<0.01). Among the seeds, PAP had greater DM, CP, NDF, ADF, ash, and ADL and lower OM and ME concentrations compared to MUC (p<0.01). Concentrations of the bioactive condensed tannins (proanthocyanidins), L-dopa, and benzyl isothiocyanate in the lespedeza hay, mucuna seeds, and papaya seeds were within normal ranges of 8.7% to 18.1% [27], 2.2% to 5.4% [28], and 2 to 687 mg/kg [29], respectively.
The DM, OM, and NDF values of BG hay were similar to those in other studies on tropical grasses [30]. However, the CP concentration was higher than observed in Foster et al [30] but similar to those reported by McCornick et al [31]. The relatively high CP value for BG hay is attributable to the fact that it was harvested in the fall as values for bahiagrass harvested in the summer at a similar regrowth interval (6 wk) are typically lower. The high CP concentration of the BG would have reduced comparative benefits of the supplementary treatments on average daily gain (ADG). Similar chemical composition to previous reports was observed for PEA [30], LES [27], MUC [32], and PAP [33].
Dry matter intake (DMI) tended (p = 0.11) to be greater in goats fed PEA and LES than those fed BG but intakes of OM and NDF did not differ among treatments (Table 2). The N intake tended (p = 0.07) to be greater in goats fed PEA, LES, and MUC than in those fed BG alone. Legume supplementation tended (p = 0.07) to increase N intake due to the greater CP concentrations of PEA, LES, and MUC vs BG. These results were consistent with previous reports on legume supplementation of grass-based diets [30]. Despite having the greatest CP concentration, N intake was not increased by PAP due to the small amount of PAP supplemented (10 g/d). The ADL intake was greater in goats (p<0.01) fed LES and PEA than the other treatments due to the relatively high concentrations of ADL in the hays.
Apparent digestibilities of DM and OM were greater (p <0.01) in goats fed MUC than BG (Table 3) perhaps reflecting a greater supply of fermentable carbohydrates for microbial growth by the starch in MUC [32]. The NDF digestibility tended (p = 0.07) to be less in LES than in MUC and BG. This is partly attributable to the greater ADL intake on the LES diet as high lignin concentrations limit forage digestibility. The N digestibility was greater (p<0.01) in MUC and PEA diets than LES, BG, or PAP. Studies have shown that M. pruriens supplementation has increased N intake and retention, weight gain and milk production in ruminants [34]. Supplementation with PEA hay has also increased N digestibility and retention in lambs [30]. Tannins in LES may have contributed to the low NDF and N digestibility of the LES diet as they form indigestible complexes with protein and carbohydrates that reduce digestibility [35]. No differences in N retention were detected among treatments (p = 0.87) because of the relatively high CP concentration of BG, the small differences in N intake among treatments and the similar N losses in urine and feces (Table 4; p>0.07).
Goats fed MUC had a lower (p<0.01) ruminal pH than all other goats (Table 5). Ruminal ammonia-N concentration was greatest (p<0.01) in goats fed MUC, followed by PEA and LES, whereas goats fed BG had the least value. Proportions of acetic (A), propionic (P), butyric, isobutyric, isovaleric, and valeric acids, total VFA concentration, and A:P ratio were not different among dietary treatments (p>0.13).
The total VFA concentrations were at the lower end of the normal range (100 to 120 mM) in forage fed ruminants partly due to the relatively low digestibilities of the diets. Ruminal pH was more acidic in goats fed MUC likely reflecting the greater starch ingestion and fermentation on the MUC diet as starch concentrations of MUC range from 27% to 31% [32], whereas they are typically less than 10% in the other ingredients that were fed [36]. Though lower than others, the ruminal pH of goats fed MUC was within the range (6.2 to 7.2) required to maintain normal cellulolytic activity in the rumen [37,38]. Legume supplementation resulted in greater ruminal ammonia-N concentrations because it tended to increase N intake compared to BG and PAP and most of the protein present in legumes is rumen-degradable. For maximum microbial N production in the rumen, an ammonia concentration of at least 5 mg/dL is recommended, though the limiting concentration is approximately 2 mg/dL [39]. Feeding MUC provided sufficient ammonia for optimizing microbial N synthesis and feeding the other supplements ensured the concentrations were not limiting. Feeding BG alone resulted in insufficient ammonia-N for optimizing microbial N synthesis.
There were no differences in BUN or plasma glucose concentration among the treat-ments (Table 5; p>0.31) but the respective values were within the normal physiological ranges (8 to 20 and 50 to 80 mg/dL, respectively; [40]). The fact that supplementation with PEA, LES, and MUC increased ammonia-N but not BUN suggests that fermentable energy supply from these diets was adequate in relation to the effective ruminally degradable protein supplied.
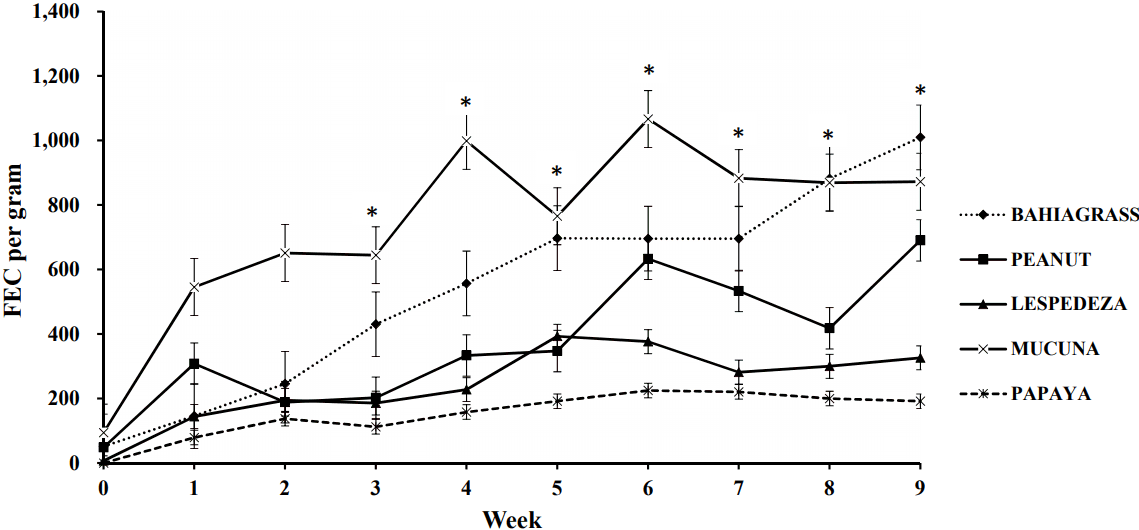
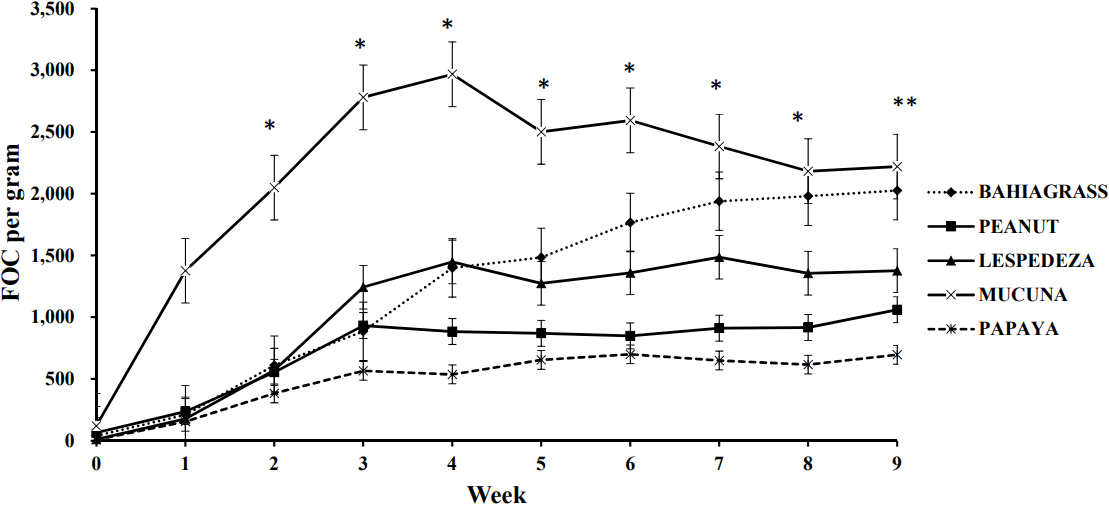
Interactions between treatment and week were observed for both FEC and FOC (Table 6; p<0.02). As expected, FEC values started to increase after approximately 3 weeks of exposure to GIN [41]. From wk 3 to 5 and 8 to 9, goats fed BG and MUC had higher FEC than the other treatments (Figure 1). After wk 3, goats fed PAP consistently had lower values than those fed other treatments except LES. Values for goats fed PAP and LES were similar except at wk 5, 6, 8, and 9, when values for PAP were lower. Goats fed PEA had lower FEC than those fed BG at wk 3, 4, 5, 8, and 9. In the case of FOC, goats fed MUC had higher counts than those fed BG from wk 1 to 6 (Figure 2) but those fed PAP and PEA, had lower values than BG from wk 4 to 9. Goats fed LES only had lower FOC than those fed BG in wk 7 to 9. Across the experimental period, goats fed PAP and LES reduced FEC (p<0.01) by 72% and 55%, respectively relative to those fed BG (Table 6), whereas feeding PEA caused a numerical reduction (p>0.05; 32%). For FOC, goats fed PAP, PEA, and LES had fewer (p = 0.02) counts than those fed MUC, but similar values to those fed BG when averaged across the experimental period. Feeding MUC did not affect abomasal adult worm counts (p = 0.69) but feeding PAP, LES, and PEA (p<0.001) reduced their presence in the abomasum by 78%, 52%, and 41%, respectively, vs BG (Table 6).
Relative to values for BG, the higher CP concentrations of LES and PEA would have increased their nutritional status and possibly made the goats more resilient to the parasites [42]. Furthermore, the presence of proanthocyanidins (i.e. condensed tannins) in LES (11.4% of DM, Table 1) may explain the superior anthelmintic effects of LES relative to PEA. The LES proanthocyanadin concentration in this study was higher than the range observed (4.8% to 9.5%) by Muir et al [43] for the same variety grown in the southeastern U.S. However, tough the condensed tannin extraction procedure as the same for this present study and that of Muir et al [43], proanthocyanadins were measured as condensed tannins with a spectrophotometer-based technique by Muir et al [43] rather than the HPLC-based technique used in this study. Several studies have reported that LES intake reduced FEC and larval development in small ruminants. Intake of LES has also reduced the adult worm burden by up to 78% [44] and decreased adult female fecundity in the abomasum and small intestine of goats. In contrast, no published studies showing anthelmintic effects of PEA were found in the literature though its nutritional benefits are well documented [30]. More research is required to support these findings and to determine the optimal level of PEA supplementation for controlling GIN in goats.
The most effective treatment against FEC and abomasal adult worm counts in this study was PAP. Previous studies demonstrated the anthelmintic efficacy of papaya extracts or seeds against GIN in sheep [45]. This effect has been attributed to compounds in PAP such as alkaloids carpaine and carpasemin or to cysteine proteases or benzyl isothiocyanate. Based on chromatographic quantification of these compounds and anthelmintic tests, benzyl isothiocyanate was reported to be the major source of the anthelmintic activity in papaya seeds [46]. Because benzyl isothiocyanate is potentially toxic, goitrogenic, carcinogenic, and mutagenic, great care is needed to prevent an overdose with papaya seeds in ruminants. Previous reports in animals and humans suggest that non-lethal amounts should be between 1 to 6.2 g of seeds/d or 56 to 112 mg/kg of BW according to the species [47]. However, doses of up to 15 g/head/d are typically used in some parts of South America (Fernando Carcel├®n, personal communication). In this study, the dose of 10 g/head/d was used to maximize the anthelmintic potential and no adverse effects of this dose on voluntary intake, growth, or health of the goats was observed. This daily dose supplied 4.38 mg of benzyl isothiocyanate to each goat in the study (Table 1).
Few studies have examined the effect of the supplements used in this study on coccidian infections. Lin et al [48] also reported that FOC in goats were reduced by feeding leucaena (Leucaena leucocephala), which contains 0.51% to 1.60% of DM condensed tannins. Condensed tannins may explain why supplementation with LES decreased FOC, FEC and abomasal adult worm counts in this study. However, since the PEA in this study did not have detectable levels of condensed tannins, the reductions in parasite counts may be due to indirect beneficial effects of the improved nutritional status on the immunity of the goats.
Previous studies revealed that papaya leaves exhibited anti coccidian properties [49]. This study indicates that papaya seeds can also be strategically used to decrease coccidian FOC in goats. Future research should confirm these findings and examine the optimal dietary inclusion rate of PAP for this purpose.
Although MUC have relatively high CP concentrations (25% to 35% of DM) and feeding MUC at 24% of the diet DM reduced FOC scores in lambs [50], MUC did not reduce FEC or FOC in this study. This may be because of differences in the variety and inclusion rates of MUC in both studies. The reason for increased FEC and FOC when feeding MUC at certain periods in the trial is unknown but it may be related to antinutrients in MUC such as the L- dopa. Although 53% of mucuna L-dopa can be ruminally degraded, residual amounts may be sufficient to compromise the immune response as in non-ruminants [51]. This may have facilitated the growth of GIN and coccidia in the parasitized goats. Anthelmintic properties of MUC may be confined to trichomes of MUC which contain mucunaine, a cysteine protease that may damage intestinal nematodes [52] and thus control GIN infections. Future studies should examine the effects of feeding MUC trichomes and seeds at different dietary inclusion levels on GIN and coccidian parasite burdens.
There were no differences in hematocrit values and FAMACHA scores among treatments (Table 7), indicating that treatments did not affect anemia indices. Previous studies reported that GIN infections and the attendant anemia can generally be alleviated by feeding sufficient supplementary protein [41]. Goats received 150 g of corn and soybean concentrate as well as forages that contained at least 12% CP daily during the evaluation period. Therefore, the high protein concentration of the diets likely prevented anemia.
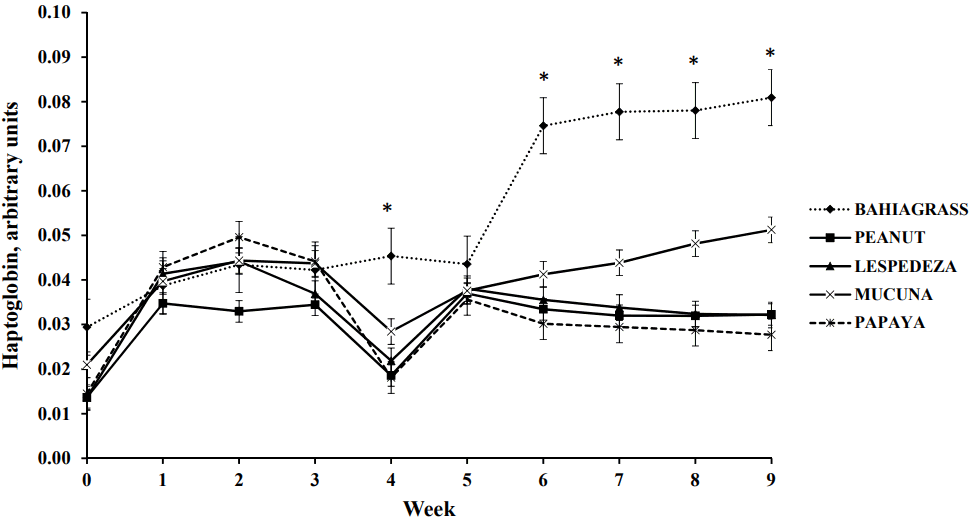
Haptoglobin is a hemoglobin-binding protein, which can be used to monitor activation of the acute phase response during an infectious disease, to assess the health status of cattle or to identify immunocompromised animals. Goats fed BG had the greatest haptoglobin concentrations on average, whereas those fed PEA and PAP had the lowest values. Nevertheless, effects of treatments on haptoglobin concentration varied with time (p<0.01; Figure 3). Mean haptoglobin concentrations were similar from wk 0 to 3 and 5, after which those of goats fed BG increased dramatically and remained greater than those of other goats till the end of the trial. Goats fed MUC also had greater haptoglobin concentrations than others in the last 3 weeks of the trial. Therefore, goats fed BG and MUC, which had the greatest FEC counts, also had greater haptoglobin concentrations. This suggests that greater levels of parasitism in the goats compromised their health and elicited an inflammatory response against the infection. In contrast, due to the anthelmintic effect of PEA, PAP, and LES no inflammatory response was elicited when they were fed and consequently they did not have elevated haptoglobin concentrations.
Goats fed PAP, PEA, and LES had greater DMI (p<0.01; Table 8) than those fed BG and those fed MUC had intermediate values. Higher FEC and abomasal adult worm counts were also detected for BG and MUC relative to the other treatments. As previously reported, the associated anorexia reported in parasitized animals is the major factor affecting performance [53].
There were no differences among treatments in initial or final BW, ADG, or gain:feed ratio among treatments (p>0.23), which may be related to the supply of sufficient CP for growth in all dietary treatments as well as the relatively low level of GIN infection in the study. The experiment was conducted from January to April, when GIN parasite infection levels are less than those in the summer and fall. Future experiments should examine the anthelmintic effects of the supplements tested in this study against severe cases of GIN parasitosis in the summer.
Feeding MUC increased digestibility of DM, OM, NDF, and N but did not reduce GIN infection or increase the performance of goats. Feeding PAP, PEA, and LES increased DMI but did not increase the performance of goats. Nevertheless, PAP, LES, and PEA reduced abomasal adult worm counts by 78%, 52%, and 41%, respectively. In particular, this study confirmed that LES is an effective anthelmintic against GIN but is less effective against coccidia. Perhaps for the first time, this study showed that PEA could be used to decrease the GIN and coccidia burden of small ruminants. More research is needed to confirm the efficacy of using PEA as a dewormer and to determine the optimal inclusion rate in small ruminant diets. PAP was the most effective anthelmintic treatment in this study and it is readily available in tropical and subtropical regions. This factor and the low dose required for efficacy indicates that PAP may be a promising natural antiparasite for controlling GIN and coccidia infections. Future research should determine the optimal doses and relative economic value of using PAP, PEA, and LES as antihelmintics and investigate their long-term effects on small ruminants and other species.
ACKNOWLEDGMENTS
We gratefully acknowledge assistance with proanthocyanidin and benzyl isothiocyanate analyses by Drs. Liwei Gu, Kari Basso, and Maria Christina Dancel of the University of Florida Department of Food Science and Human Nutrition and the Department of Chemistry, Mass Spectrometry services, respectively. We are also grateful to Dr. Charles Courtney and Antoinette McIntosh, Infectious Diseases and Pathology, College of Veterinary Medicine, University of Florida, for advice about the design of the study and assistance with the parasitology and hematocrit assays, respectively. This study was funded by a USDA-TSTAR grant.
Figure┬Ā1
Effects of supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP) on gastrointestinal fecal egg counts (FEC). Treatment├Śtime, p<0.05. Error bars are standard errors. * Counts at the week specified differed (p<0.05).
Figure┬Ā2
Effects of supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP) on coccidian fecal oocysts counts (FOC). Treatment├Śtime, p<0.05. Error bars are standard errors. * Counts at the week specified differed (p<0.05) and ** counts at the week specified tended to differ (p<0.1).
Figure┬Ā3
Effects of supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP) on plasma haptoglobin concentrations. Treatment├Śtime, p<0.05. Error bars are standard errors. * Counts at the week specified differed (p<0.05).
Table┬Ā1
Chemical composition of the bahiagrass (BG), perennial peanut (PEA), sericea lespedeza (LES) hays, mucuna (MUC), and papaya (PAP) seeds fed to goats (n = 7)
| Measure | BG | PEA | LES | MUC | PAP | SEM | p-value |
|---|---|---|---|---|---|---|---|
| DM (%) | 90.2b | 89.1a | 89.9b | 90.4b | 92.9c | 0.3 | <0.01 |
| OM (% of DM) | 94.6c | 91.8b | 95.4d | 96.2e | 90.8a | 0.1 | <0.01 |
| NDF (% of DM) | 72.8e | 51.9c | 60.9d | 13.5a | 32.3b | 0.4 | <0.01 |
| ADF (% of DM) | 34.7c | 36.6d | 46.1e | 8.7a | 27.3b | 0.2 | <0.01 |
| CP (% of DM) | 12.4a | 14.3b | 14.6b | 28.5c | 30.0d | 0.1 | <0.01 |
| N (% of DM) | 1.9a | 2.2b | 2.3b | 4.4c | 4.7d | 0.01 | <0.01 |
| Ash (% of DM) | 5.4c | 8.2d | 4.6b | 3.8a | 9.2e | 0.1 | <0.01 |
| ADL (% of DM) | 5.1c | 9.4d | 17.6e | 0.3a | 1.9b | 0.3 | <0.01 |
| ME (MJ/kg of DM) | 8.4e | 10.6c | 9.7d | 14.5a | 12.6b | 0.03 | <0.01 |
| Proanthocyanidins (% of DM) | ND | ND | 11.4 | ND | ND | NM | - |
| Benzyl isothiocyanate (mg/kg of DM) | NM | NM | NM | NM | 438 | NM | - |
| L-dopa (% of DM) | NM | NM | NM | 3.4 | NM | NM | - |
Table┬Ā2
Effects on intake of dry matter (DMI), organic matter (OMI), neutral detergent fiber (NDFI), nitrogen (NI), and acid detergent lignin (ADLI) in goats resulting from supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, or mucuna (MUC) or papaya seeds (PAP)
| Measure (g/d) | BG | PEA | LES | MUC | PAP | SEM | p-value |
|---|---|---|---|---|---|---|---|
| DMI | 612 | 757 | 745 | 627 | 627 | 89 | 0.11 |
| OMI | 580 | 710 | 702 | 596 | 593 | 89 | 0.15 |
| NDFI | 458 | 506 | 523 | 408 | 472 | 66 | 0.18 |
| NI | 12 | 16 | 15 | 15 | 12 | 2 | 0.07 |
| ADLI | 32a | 50b | 65c | 28a | 32a | 7 | <0.01 |
Table┬Ā3
Effects on in vivo apparent digestibility of dry matter (DMD), organic matter (OMD), neutral detergent fiber (NDFD), and nitrogen (ND) in goats resulting from supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, or mucuna (MUC) or papaya seeds (PAP)
| Measure (%) | BG | PEA | LES | MUC | PAP | SEM | p-value |
|---|---|---|---|---|---|---|---|
| DMD | 54.9ab | 56.5bc | 53.1ab | 59.5c | 51.1a | 1.8 | <0.01 |
| OMD | 56.0ab | 57.9bc | 53.8ab | 60.8c | 52.1a | 1.7 | <0.01 |
| NDFD | 62.8 | 59.4 | 56.4 | 61.5 | 59.8 | 1.8 | 0.07 |
| ND | 50.5a | 58.9b | 49.0a | 61.2b | 49.7a | 1.8 | <0.01 |
Table┬Ā4
Effects on nitrogen (N) balance in goats resulting from supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP)
Table┬Ā5
Effects on ruminal fermentation indices, blood urea nitrogen (BUN) and plasma glucose concentrations in goats resulting from supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP)
| Measure | BG | PEA | LES | MUC | PAP | SEM | p-value |
|---|---|---|---|---|---|---|---|
| Ruminal pH | 7.0b | 6.7b | 6.7b | 6.3a | 6.8b | 0.1 | <0.01 |
| Ammonia N (mg/dL) | 1.2a | 3.5b | 3.5b | 6.3c | 1.7ab | 0.8 | <0.01 |
| Total VFA (mM) | 89.2 | 100.1 | 88.0 | 95.2 | 98.4 | 11.7 | 0.92 |
| Acetate (A, mM) | 63.8 | 68.1 | 61.6 | 66.0 | 70.1 | 8.2 | 0.95 |
| Propionate (P, mM) | 19.2 | 22.7 | 18.9 | 19.3 | 20.2 | 3.4 | 0.94 |
| Iso-butyrate (mM) | 0.4 | 0.3 | 0.6 | 0.9 | 0.4 | 0.2 | 0.13 |
| Butyrate (mM) | 5.0 | 7.6 | 5.9 | 7.1 | 5.7 | 1.2 | 0.49 |
| Iso-valerate (mM) | 0.5 | 1.6 | 1.3 | 1.0 | 1.2 | 0.6 | 0.70 |
| Valerate (mM) | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | 0.1 | 0.50 |
| A:P | 3.6 | 3.5 | 3.7 | 3.6 | 3.6 | 0.5 | 0.99 |
| BUN (mg/dL) | 13.2 | 15.8 | 13.1 | 14.6 | 12.9 | 1.2 | 0.31 |
| Plasma glucose (mg/dL) | 70.0 | 61.6 | 62.9 | 65.6 | 63.5 | 2.1 | 0.64 |
Table┬Ā6
Effects of supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP) on gastrointestinal (GIN) fecal egg count (FEC), coccidian fecal oocysts count (FOC) and abomasal adult worm counts
| Measure | BG | PEA | LES | MUC | PAP | SEM | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Treatment | Week | Treatment├Śweek | |||||||
| FEC (eggs/g) | 541bc | 370ab | 244a | 739c | 152a | 83.3 | <0.01 | <0.01 | <0.01 |
| FOC (oocysts/g) | 1,235ab | 725a | 1,031a | 2,120b | 497a | 315.9 | 0.02 | <0.01 | 0.02 |
| Adult worm counts | 3,066c | 1,798b | 1,462b | 2,934c | 690a | 269 | <0.01 | NA | NA |
Table┬Ā7
Effects of supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP) on hematocrit, FAMACHA scores and plasma haptoglobin concentrations of goats
| Measure | BG | PEA | LES | MUC | PAP | SEM | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Treatment | Week | Treatment├Śweek | |||||||
| Hematocrit (%) | 25.4 | 24.6 | 24.9 | 23.8 | 26.1 | 1.3 | 0.56 | 0.14 | 0.87 |
| FAMACHA score | 1.36 | 1.42 | 1.34 | 1.31 | 1.06 | 0.18 | 0.65 | 0.53 | 0.79 |
| Haptoglobin, arbitrary units | 0.055c | 0.030a | 0.033ab | 0.040b | 0.032a | 0.003 | <0.01 | <0.01 | <0.01 |
Table┬Ā8
Effects of supplementing bahiagrass (BG) hay with perennial peanut (PEA), lespedeza (LES) hay, mucuna (MUC), or papaya seeds (PAP) on the performance of goats
| Measure | BG | PEA | LES | MUC | PAP | SEM | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Treatment | Week | Treatment├Śweek | |||||||
| DMI (g/head/d) | 617a | 730bc | 750c | 661ab | 775c | 52 | <0.01 | <0.01 | 0.11 |
| DMI (% of BW) | 2.26a | 2.72b | 2.78b | 2.37a | 2.62b | 0.20 | <0.01 | <0.01 | 0.51 |
| Initial BW (kg) | 27.2 | 26.8 | 26.2 | 26.8 | 27.7 | 3.69 | 0.74 | NA | NA |
| Final BW (kg) | 30.2 | 29.9 | 30.8 | 30.9 | 32.5 | 3.60 | 0.54 | NA | NA |
| ADG (g/d) | 47 | 50 | 73 | 66 | 69 | 11 | 0.23 | NA | NA |
| Gain:feed ratio | 0.55 | 0.47 | 0.68 | 0.70 | 0.69 | 0.12 | 0.39 | NA | NA |
REFERENCES
1. Pinkerton F, Escobar EN, Harwell L, Drinkwater W. A survey of prevalent production and marketing practices in meat goats of southern origin. Langston Univ Goat Res Ext Newslett 1994; 182:1ŌĆō47.
2. Miller J. Controlling goat parasites in the Southeast. In : Proceedings of the Southeast Regional Meat Goat Production Symposium: Meat Goat Production in the SoutheastŌĆöToday and Tomorrow; Tallahassee, FL, USA: Florida A&M University; 1996. p. 21ŌĆō4.
4. Foreyt WJ. Coccidiosis and cryptosporidiosis in sheep and goats. Vet Clin N Am-Food A 1990; 6:655ŌĆō70.

5. Gibbs HC, Herd RP. Nematodiasis in cattle - Importance, species involved, immunity, and resistance. Vet Clin N Am-Food A 1986; 2:211ŌĆō24.

6. Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol 2004; 20:477ŌĆō81.


7. Shaik SA, Terrill TH, Miller JE, et al. Sericea lespedeza hay as a natural deworming agent against gastrointestinal nematode infection in goats. Vet Parasitol 2006; 139:150ŌĆō7.


8. Jalalpure S, Alagawadi K, Mahajanashetti C, et al.
In vitro anthelmintic property of various seed oils against Pheritima posthuma
. Indian J Pharm Sci 2007; 69:158ŌĆō60.

9. Buttle DJ, Behnke JM, Bartley Y, et al. Oral dosing with papaya latex is an effective anthelmintic treatment for sheep infected with Haemonchus contortus
. Parasit Vectors 2011; 4:36



10. FAWN. Florida automated weather network [Internet]. University of Florida; c2012. [cited 2012 Jul 28, 2012]. Available from: http://fawn.ifas.ufl.edu
11. AOAC. Official methods of analysis. 17th edAOAC Int; 2000.
12. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 1991; 74:3583ŌĆō97.


13. Siddhuraju P, Becker K. Rapid reversed-phase high performance liquid chromatographic method for the quantification of L-Dopa (L-3,4-dihydroxyphenylalanine), non-methylated and methylated tetrahydroisoquinoline compounds from Mucuna beans. Food Chem 2001; 72:389ŌĆō94.

14. Terrill TH, Rowan AM, Douglas GB, Barry TN. Determination of extractable and bound condensed tannin concentrations in forage plants, protein-concentrate meals and cereal-grains. J Sci Food Agric 1992; 58:321ŌĆō9.

15. Buran TJ, Sandhu AK, Azeredo AM, et al. Effects of exogenous abscisic acid on fruit quality, antioxidant capacities, and phytochemical contents of southern high bush blueberries. Food Chem 2012; 132:1375ŌĆō81.


16. Muck RE, Dickerson JT. Storage-temperature effects on proteolysis in alfalfa silage. Trans ASAE 1988; 31:1005ŌĆō9.

17. Noel RJ, Hambleton LG. Collaborative study of a semiautomated method for the determination of crude protein in animal feeds. J AOAC 1976; 59:134ŌĆō40.

18. Gochman N, Schmitz JM. Application of a new peroxide indicator reaction to the specific, automated determination of glucose with glucose oxidase. Clin Chem 1972; 18:943ŌĆō50.


19. Coulombe JJ, Favreau L. A new simple semimicro method for colorimetric determination of urea. Clin Chem 1963; 9:102ŌĆō8.


20. Whitlock H. Some modifications of the McMaster helminth eggcounting technique and apparatus. J Counc Sci Ind Res 1948; 21:177ŌĆō80.
21. Makimura S, Suzuki N. Quantitative-determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Jpn J Vet Sci 1982; 44:15ŌĆō21.

22. Kaplan RM, Burke JM, Terrill TH, et al. Validation of the FAMACHA┬® eye color chart for detecting clinical anemia in sheep and goats on farms in the southern United States. Vet Parasitol 2004; 123:105ŌĆō20.


23. Hansen J, Perry B. The epidemiology, diagnosis and control of helminth parasites of ruminants. A handbook. Nairobi, Kenya: Int Livest Res Inst; 1994.
24. Miller JE, Bahirathan M, Lemarie SL, et al. Epidemiology of gastrointestinal nematode parasitism in Suffolk and Gulf coast native sheep with special emphasis on relative susceptibility to Haemonchus contortus infection. Vet Parasitol 1998; 74:55ŌĆō74.


25. NRC. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington, DC, USA: National Academies Press; 2007.
26. Mertens D. Equation standardization-Which ones to use in predicting intake and energy value. In : Proc National Invitational Near Infrared Reflectance Spectroscopy Workshop; 1985. Madison, WI, USA: Extension Committee on Programs.
27. Terrill TH, Windham WR, Hoveland CS, Amos HE. Forage preservation method influences on tannin concentration, intake, and digestibility of Sericea lespedeza by sheep. Agron J 1989; 81:435ŌĆō9.

28. Raina AP, Khatri R. Quantitative determination of L-DOPA in seeds of Mucuna pruriens germplasm by high performance thin layer chromatography. Indian J Pharm Sci 2011; 73:459ŌĆō62.


29. Nakamura Y, Yoshimoto M, Murata Y, et al. Papaya seed represents a rich source of biologically active isothiocyanate. J Agric Food Chem 2007; 55:4407ŌĆō13.


30. Foster JL, Adesogan AT, Carter JN, et al. Intake, digestibility, and nitrogen retention by sheep supplemented with warm-season legume hays or soybean meal. J Anim Sci 2009; 87:2891ŌĆō8.


31. McCormick ME, Han KJ, Moreira VR, Blouin DC, Forbes S. Forage conservation efficiency and lactation response to bahiagrass conserved as barn-stored hay, outdoor-stored hay, or baleage. J Dairy Sci 2011; 94:2500ŌĆō7.


32. Chikagwa-Malunga SK, Adesogan AT, Salawu MB, et al. Nutritional characterization of Mucuna pruiriens: 2. In vitro ruminal fluid fermentability of Mucuna pruriens, Mucuna l-dopa and soybean meal incubated with or without l-dopa. Anim Feed Sci Technol 2009; 148:51ŌĆō67.

33. Puangsri I, Abdulkarim SM, Ghazali HM. Properties of Carica papaya L. (papaya) seed oil following extractions using solvent and aqueous enzymatic methods. J Food Lipids 2005; 12:62ŌĆō76.

34. Castillo-Caamal J, Jimenez-Osornio J, Lopez-Perez A, Aguilar-Cordero W, Castillo-Caamal A. Feeding Mucuna beans to small ruminants of Mayan farmers in the Yucatan Peninsula, Mexico. Trop Subtrop Agroecosyst 2003; 1:113ŌĆō7.
35. Min BR, Hart SP. Tannins for suppression of internal parasites. J Anim Sci 2003; 81:E102ŌĆōE9.
36. Egli DB, Leggett JE, Cheniae A. Carbohydrate levels in soybean leaves during reproductive growth. Crop Sci 1980; 20:468ŌĆō73.

37. Van Soest PJ. Nutritional ecology of the ruminant. 2nd edIthaca, NY, USA: Cornell University Press; 1994.
38. Grant RH, Mertens DR. Influence of buffer pH and raw corn starch addition on in vitro fiber digestion kinetics. J Dairy Sci 1992; 75:2762ŌĆō8.


39. Satter LD, Slyter LL. Effect of ammonia concentration on rumen microbial protein production in vitro
. Br J Nutr 1974; 32:199ŌĆō208.


40. Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals. Burlington, MA, USA: Academic Press; 2008.
41. Sutherland I, Scott I. Gastrointestinal nematodes of sheep and cattle: biology and control. 1st edChichester, West Sussex, UK: John Wiley & Sons; 2009.
42. Min BR, Pomroy WE, Hart SP, Sahlu T. The effect of short-term consumption of a forage containing condensed tannins on gastro-intestinal nematode parasite infections in grazing wether goats. Small Rumin Res 2004; 51:279ŌĆō83.

43. Muir JP, Terrill TH, Kamisetti NR, Bow JR. Environment, harvest regimen, and ontogeny change lespedeza cuneata condensed tannin and nitrogen. Crop Sci 2014; 54:2903ŌĆō9.

44. Min BR, Hart SP, Miller D, et al. The effect of grazing forage containing condensed tannins on gastro-intestinal parasite infection and milk composition in Angora does. Vet Parasitol 2005; 130:105ŌĆō13.


45. Ameen S, Adedeji O, Ojedapo L, Salihu T, Fabusuyi C. Anthelmintic potency of pawpaw (Carica papaya) seeds in West African Dwarf (WAD) sheep. Glob Vet 2010; 5:30ŌĆō4.
46. Kermanshai R, McCarry BE, Rosenfeld J, et al. Benzyl isothiocyanate is the chief or sole anthelmintic in papaya seed extracts. Phytochemistry 2001; 57:427ŌĆō35.


47. Roig y Mesa JT. Medicinal, aromatic, and poisonous plants of Cuba. La Habana, Cuba: Ciencia y T├®cnica; 1974.
48. Lin NK, Preston T, Van Binh D, Ly ND. Effects of tree foliages compared with grasses on growth and intestinal nematode infestation in confined goats. Livest Res Rural Dev 2003; 15:Article #41
49. Adiwimarta K, Daryatmo J, Orskov ER, Mayes RW, Hartadi H. Utilisation of cassava leaf and Carica papaya leaf as feeds and anthelmintics for goats. Adv Anim Biosci 2010; 1:114

50. Chikagwa-Malunga SK, Adesogan AT, Szabo NJ, et al. Nutritional characterization of Mucuna pruriens: 3. Effect of replacing soybean meal with Mucuna on intake, digestibility, N balance and microbial protein synthesis in sheep. Anim Feed Sci Technol 2009; 148:107ŌĆō23.

51. Del Carmen J, Gernat AG, Myhrman R, Carew LB. Evaluation of raw and heated velvet beans (Mucuna pruriens) as feed ingredients for broilers. Poult Sci 1999; 78:866ŌĆō72.



52. Conroy C, Thakur Y. A local plant for de-worming goats. LEISA 2005; 21:24






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





