 |
 |
| Anim Biosci > Volume 30(10); 2017 > Article |
|
Abstract
Objective
Our study provides information on phenotypes of local chickens and guinea fowl and their body measures as well as on major genes in local chickens in northern Ghana.
Methods
Qualitative and morphometric traits were recorded on 788 local chickens and 394 guinea fowl in urban households in Tamale, Ghana.
Results
The results showed considerable variation of color traits and numerous major genes in local chickens, while color variations and related genotypes in guinea fowl were limited. In local chickens, white was preferred for plumage, whereas dark colors were preferred for beak and shanks. More than half of the chickens carried at least one major gene, but the contributions of single gene carriers were low. All calculated allele frequencies were significantly lower than their expected Mendelian allele frequencies. We observed higher mean body weight and larger linear body measures in male as compared to female chickens. In female chickens, we detected a small effect of major genes on body weight and chest circumference. In addition, we found some association between feather type and plumage color. In guinea fowl, seven distinct plumage colors were observed, of which pearl grey pied and pearl grey were the most prevalent. Male pearl grey pied guinea fowl were inferior to pearl grey and white guinea fowl in terms of body weight, body length and chest circumference; their shank length was lower than that of pearl grey fowl.
Conclusion
Considerable variation in qualitative traits of local chickens may be indicative of genetic diversity within local chicken populations, but major genes were rare. In contrast, phenotypic and genetic diversity in local guinea fowl is limited. Broader genetic diversity studies and evaluation of trait preferences of local poultry producers are required for the design of appropriate breeding programs.
In developing countries, investments and policies are mainly centered on production systems using exotic breeds [1], while local poultry breeds are often ignored due to their lower performances. This has raised concerns of loss of poultry genetic resources in many countries. Furthermore, the narrowing genetic base in commercial breeds and the use of a few breeds of poultry in intensive production systems is precipitating the loss of fringe breeds [1]. According to Okantah et al [2], the rapid population growth in Ghana has led to a higher importance of highly selected poultry birds to meet the growing demand for meat and eggs. Nevertheless, local poultry species are still prominent in the Northern region of Ghana as they, together with livestock, make vital contributions to households and enterprises [3].
Local chicken breeds are often endowed with major genes that confer adaptability and improve performances or immunocompetence in tropical conditions [4,5]. Low frequencies of major gene carriers among local chickens however strongly suggest that major genes may be on the brink of extinction [6]. In Kumasi in Southern Ghana, a breeding program that aims at developing a highly adapted chicken breed from naked neck and frizzle phenotypes has been initiated [7]. Besides, no additional information on breeding or conservation of local poultry resources in Ghana is available. Furthermore, few research efforts were undertaken to assess the diversity of local poultry resources and to characterize local chickens either phenotypically or genetically. Characterization of local animal genetic resources is a first step in making available the required information for the use and conservation of animal genetic resources [8]. In this context, it is important to understand the role of major genes and to determine their contribution to local chicken populations. The objectives of the present study were therefore i) to provide basic information about the diversity of different phenotypes of local chickens and guinea fowl, ii) to examine the occurrence of major gene carriers among local chickens, and iii) to assess the production potential of different local chicken and guinea fowl phenotypes under local husbandry conditions in urban production systems.
The study was conducted in 20 (peri-) urban communities in Tamale Metropolis from April 27 to September 18, 2015. Tamale Metropolis is the third largest settlement in Ghana. The study area lies between latitudes 9°16 and 9°34 North and longitudes 0°36 and 0°57 West. It belongs to the Guinea Savannah ecological zone and experiences a unimodal annual rainfall of 750 to 1,050 mm [9]. The rainy season typically begins in April/May and ends in October/November; the subsequent dry season spans from October/November to March. Maximum daily temperatures are 32°C to 43°C whereas minimum temperatures range from 19°C to 23°C [10].
This study was conducted in the frame of the UrbanFoodPlus project, an African-German partnership to enhance resource use efficiency and improve food security in urban and peri-urban agriculture of West African cities. In 2014, the livestock subproject of the above mentioned research project realized a baseline survey of 187 livestock-keeping households in and around Tamale [11]. The households that had at least 10 chickens and/or guinea fowl (n = 77) were selected for the present study. One additional household that was not part of the baseline survey was also included. The majority of these households (98.7%) kept poultry for income generation and home consumption. The majority of poultry owners (84.6%) had traditional knowledge, while only 15.4% received some form of technical training by public authorities. The major part (70.5%) owned poultry for more than 20 years, 15.4% for 11 to 20 years, while a minor proportion had experiences for 6 to 10 years (9.0%) and 1 to 5 years (5.1%), respectively. All households owned local chickens and 46.2% additionally owned guinea fowl. The average flock sizes per household were 26.9±15.84 chickens (minimum: 6, maximum: 100) and 16.3± 19.32 guinea fowl (minimum: 0, maximum: 115). All adult (10 to 13 months) local chickens and guinea fowl that were present at the time of visit were included in the study, resulting in 788 local chickens (136 cocks, 652 hens) and 394 guinea fowl (121 cocks, 273 hens). The average number of local chickens assessed per farm amounted to 10.2±5.15 birds (minimum: 3, maximum: 33), and that of guinea fowl amounted to 9.9±5.17 birds (minimum: 1, maximum 20).
The studied chickens and guinea fowl were kept under similar management conditions in extensive, scavenging systems. Birds roamed and scavenged for feed during the day and were confined in various structures during the night. Housing structures were generally poor (71.7%) and management assets such as brooding lamps (1.3%), laying nests (9.0%), and troughs (5.1%) were rarely used. Most of the poultry owners (85.9%) used baskets, wooden cages or improvised materials for confinement of poultry. Supplement feed (agro by-products and kitchen wastes, 98.7%) and water were provided daily. Nearly all households (94.9%) did not use extension service in the last 12 months. Similarly, 87.2% did not use veterinary service.
Qualitative traits were assessed through individual visual observation of all birds for various phenotypic variables as described in [12], including plumage, beak, eye, comb, skin and shank color. In addition, helmet and head cap color were assessed in guinea fowl, and earlobe color, comb type (normal, rose, and pea comb), feather distribution (normal, naked neck, ptilopody, and crested head) and feather structure (normal, frizzled, and silky feather) and polydactyly were recorded in local chickens. Shank, earlobe and eye colors as well as comb types were identified based on pictorial descriptions provided by Cuesta [13]. All qualitative assessments were subjected to a second observation based on photographs of each bird.
Morphometric traits were measured according to the FAO guidelines for phenotypic characterization of animal genetic resources [12]. Traits included body weight, body length (length between the tip of the beak and that of the tail (without feathers), chest circumference (taken at the tip of the hind breast), shank length (length between the hock joint to the spur) and wingspan (length between tips of right and left wings after both stretched out in full). Body weight was assessed in kg using a portable electronic hanging scale (5 g precision) (Guangzhou Weiheng Electronics Co. Ltd., Guangzhou, China), and linear body measures were taken to the nearest 0.5 cm using a plastic tailoring tape measure.
SPSS 20.0 (SPSS Inc., an IBM Company Chicago, IL, USA) was used for statistical analyses. Occurrence and distribution of qualitative traits were calculated. To determine the effect of individual households and the sex of the bird on qualitative traits, we used the Kruskal-Wallis test. We observed no significant effect for the distribution of major gene carriers in local chicken flocks between individual households and between hens and cocks (p>0.05), respectively. Data of major gene carriers in the 788 local chickens were therefore pooled to calculate allele frequencies. Observed allele frequencies (p of dominant alleles, q of recessive alleles) were calculated for major genes in local chickens (naked neck, Na/na; frizzled feather, F/f; rose comb, R/r; pea comb, P/p; silky feather, H/h; crested head, Cr/cr; ptilopody, Pti/pti; polydactyly, Po/po) using the Hardy-Weinberg equilibrium:
q = m t
In the studied guinea fowl, we estimated the proportions of observed genotypes of color patterns using the genotypes described in [14]. We observed a significant effect of the individual flock on the observed genotypes, helmet, eye and head cap skin colors (p<0.001), but not on beak colors (p>0.05). In addition, the sex of the bird influenced the observed genotypes and eye colors (p<0.05). Therefore, we compared the mean distribution of genotypes for individual flocks, separately for hens and cocks. For the analysis of average distribution of color patterns in individual flocks, we pooled the data of hens and cocks.
Morphometric traits were tested for normality with the Shapiro-Wilk’s test (p<0.05) and by visual inspection of the histograms. Levene’s test was used to confirm homogeneity of variances (p> 0.05). Sex was a significant source of variation for all morphometric traits measured in local chickens with significantly higher body weight and linear body measures obtained for cocks as compared to hens (p<0.001). In guinea fowl, three of the five morphometric traits were significantly different between males and females. Therefore, descriptive statistics (medians, means and standard error of the means) of morphometric traits were calculated separately for hens and cocks in both poultry species. Groups within subsets that had comparatively small sample sizes were excluded from further analysis. These were rose comb, ptilopody and polydactyly for female chickens, pea comb, adaptive gene combinations, crested head and polydactyly for male chickens as well as brown, light grey, sky blue and bronze for both male and female guinea fowl. Due to small and unequal sizes of phenotypic groups as well as partly non-normally distributed data, non-parametric Kruskal-Wallis H test was performed to compare mean ranks of morphometric traits between different phenotypes. In the case of significant Kruskal-Wallis H test, Dunn-Bonferroni test was used for pairwise comparisons. Significance levels used for Kruskal-Wallis H and Dunn-Bonferroni tests were p<0.05.
In total, more than half of the described local chickens carried at least one major gene, but the contributions of single genes to local chicken resources were relatively low (Figure 1). In general, major genes were more common among female chickens (57.8%), while 66.2% of the male chickens exhibited none of the adaptive genes (p<0.001). The occurrence of crested head, rose comb and pea comb was significantly different between male and female chickens (p<0.001). Crested head and pea comb were more frequent in female than in male chickens, while rose combs occurred at higher frequency in male chickens. About one quarter (22.6%) of major gene carriers exhibited more than one phenotype. The highest overall proportions were observed for crested head, pea comb, silky feathers, frizzle feathers and naked neck. Ptilopody was the least frequently found phenotype among local chickens. Consequently, the calculated allele frequencies for adaptive genes were significantly lower (p<0.01) than their respective expected Mendelian allele frequencies, being highest for crested head (0.164). For all other genes, they were below 0.06 (Table 1).
In total, seven different main plumage colors were observed (Table 2). The feathers of local chickens were mostly unicolor. White feathered hens and cocks were present in 87% and 57.6% of the household flocks, respectively, followed by black feathered chickens that were present in 74.0% (hens) and 52.5% (cocks) of the flocks. The average share of white feathered hens and cocks in individual flocks amounted to 33.4% and 37.4% (p>0.05). Black plumage was more common in cocks than in hens (p<0.05), whereas poultry owners were more likely to keep brown hens than brown cocks (p<0.001), with a significantly higher average share of brown hens in individual flocks as compared to brown cocks. Mottled plumage occurred more frequently in hens than in cocks (p<0.05). Mottled hens were found in 64.9% of the flocks, whereas mottled cocks were limited to 16.9% of household flocks (p<0.001). A higher proportion of silky feathered chickens had white feathers (75.8% vs 31.0% to 47.1% for normal chickens and other adaptive gene carriers; p<0.001). No further differences were observed for the remaining plumage colors between the studied chicken phenotypes (p>0.05).
For shanks, beaks and earlobes, five different colors were iden tified each (Table 2). While shanks of hens were predominantly black/grey (p<0.001), cocks more frequently had white (p<0.05) or yellow shanks (p<0.001). Significant differences were also observed for white earlobes that were more frequent in cocks than in hens (p<0.05). The distribution of earlobe color also differed between major gene carriers (p<0.001). The highest proportion of grey earlobes was observed for naked neck chickens (40% vs 0% to 28.1% for other phenotypes; p<0.001), while red earlobes were more frequently observed in frizzle and silky feathered chickens (47.1% and 48.5% vs 25.0% to 31.3% for other phenotypes; p<0.05). In addition, a significantly higher number of silky feathered chickens had a white beak (51.5% vs 18.8% to 35.3% for other phenotypes; p<0.001). The distribution of single beak colors was not affected by the sex (p>0.05).
We identified four eye colors with a clear preference for orange and yellow eyes (Table 2), with no differences between hens and cocks (p>0.05). In contrast, red eyes were more frequent in cocks than in hens (p<0.05), although found in a lower number of flocks as compared to red-eyed hens.
The comb and skin color were the least variable, with most of the investigated chickens having a red comb and white skin (Table 2). All household flocks had at least one hen or cock with a red comb, but the mean proportion of cocks with red comb in individual flocks was higher than that of hens (p<0.001). In contrast, hens with black comb were more frequent in individual household flocks than cocks with the same feature (p<0.001), and are found in nearly half of the studied flocks. No differences were observed for the distribution of skin colors in individual household flocks between hens and cocks (p>0.05). No relationship was observed between the eye, comb or skin color and the phenotype of local chickens (p>0.05).
In hens, the phenotype significantly influenced the body weight and chest circumference. Hens that carried more than one major gene were lighter than normal hens (z = −3.762, p<0.01). In addition, they had a smaller chest circumference than normal and crested head hens (z = −4.403, p<0.001; z = 3.844, p<0.01). In contrast, body length, wingspan and shank length did not differ significantly between female phenotypes (p>0.05) (Table 3).
Unlike hens, the phenotype had no effect on morphometric traits of cocks (p>0.05). Mean body weights ranged from 0.92 kg for frizzle feather to 1.09 kg in rose comb cocks. Mean shank and body length as well as mean wingspan were lowest in naked neck as compared to all other phenotypes for cocks (p>0.05) (Table 4).
Color distribution in local guinea fowl was less variable than in local chickens (Figure 2). Again, we observed the highest color diversity for the plumage, for which five known genotypes were identified (Table 5). Pearl grey pied and pearl grey were the most frequent phenotypes, while white, brown, light grey, light blue and bronze birds were rarely observed. The average proportion of pearl grey pied cocks in individual flocks was higher than that of pearl grey pied hens (p<0.001).
Beaks were uniformly brown and the predominant head cap skin color among the studied guinea fowl was white. Birds with white head cap skin were found in 97.1% of the household flocks, representing on average 85.6% of birds in individual flocks (Figure 2). We also observed guinea fowl with brown (40.0%) and greyish (31.4%) head cap skin, respectively; yet, the average share of these colors in individual flocks were significantly lower as compared to white. Helmet and skin colors were mainly purple. Guinea fowl with purple helmet were identified on 91.4% of the flocks, representing 57.7% of birds in individual flocks. For purple skin, the proportions were 85.7% of the flocks and 63.8% of the birds in individual flocks. The two main eye colors were black and brown (88.6% of flocks and 55.7% of birds for black eyes; 68.6% of flocks and 40.0% for brown eyes). For shanks, black (88.6% of flocks), orange (77.1% of flocks) or multicolor (purple with orange; 85.7% of flocks) was observed, while purple was rare (48.6% of flocks).
Sex differences in guinea fowl were observed for body and shank length as well as for wingspan (p<0.001). In contrast, phenotype had no effect (p>0.05) on morphometric traits of female guinea fowl (Table 6). Unlike females, all morphometric traits except for shank length were significantly different (p<0.01) among male guinea fowl of the three main phenotypes (Table 7). Pearl grey pied cocks were significantly lighter than pearl grey (z = −3.203, p<0.01) and white cocks (z = −2.646, p<0.05). In addition, their body length was smaller than that of pearl grey (z = −3.113, p< 0.01) and white cocks (z = −2.964, p<0.01). Finally, pearl grey pied male guinea fowl had a significantly smaller chest circumference (z = −3.150, p<0.01) and wingspan (z = −2.478, p<0.05) than their pearl grey counterparts.
The frequencies of local chickens carrying major genes were low, which agrees with other findings [6]. Despite their thermoregulatory relevance, frizzle feather and naked neck phenotypes were rarely found in local chicken flocks in Tamale, which corresponds to other reports from Nigeria [15]. However, the proportions in our study were higher as compared to those reported in the Domestic Animal Diversity information system [16] for total Ghana, which might be indicative of a higher importance of these phenotypes in the warmer and less humid climate in the northern part of the country. Crested head occurred in a relatively higher frequency in the studied chickens, being more frequent in female local chickens, whereas rose comb was more frequent in male local chickens, an observation shared by Dahloum et al [6]. The low frequency of pea comb in our sample could be due to its irrelevance in tropical climate as it is an adaptive trait to cold climate where it reduces heat loss [17].
Calculated allele frequencies for major genes were lower than their expected Mendelian values. The same was reported for Nigerian [16] and Algerian [6] local chicken populations. As per respondents’ declaration, ‘abnormal’ looking birds are deliberately removed from flocks as they are frown upon by society and have no market value which concurred with findings of Yakubu [16]. This practice may have contributed to the rare occurrence of other major genes in the studied flocks.
In addition, major genes did not confer performance advantages in body weight and body measures in the present study. Similarly, Hagan et al [18] did not find any effect of naked neck and frizzle gene on body weight and other growth traits in crossbred exotic and indigenous chickens in Ghana. In contrast, Oguntunji et al [19] assessed higher body weights in polydactylous than in normal, ptilopod and frizzled cocks. In general, the values for mean body and shank length of local chickens in Tamale were comparable to those given in [5]. In contrast, mean body weight and chest circumference were lower than the values reported by other authors [5,6]. Higher body weight and measures observed in male as compared to female local chickens were due to sexual dimorphism caused by different hormonal actions that invariably led to different body weights in adult male and female birds [20].
White was the most frequent skin, plumage and earlobe color in local chickens, which might be due to better tolerance of heat stress as opposed to black phenotypic characteristics [21]. A prevalence of white plumage was also reported for Savannah chicken ecotypes in Benin [22], while for local chickens in other tropical countries white plumage was less frequent [21,23]. The high proportion of white plumage birds in the present study might also be attributed to their higher prices and frequent use in religious sacrifices [22], which agrees with our respondents’ assertion that white birds command highest market prices and traditional value. Despite the preference for white birds and the observed association between white plumage and silky feathered birds, the occurrence of silky feathered chickens and the calculated allele frequency for this gene was comparatively low in the studied flocks. Besides white as most frequent earlobe color, we observed a variety of other earlobe colors. According to Negassa et al [24], the variation of earlobe colors suggests the existence of chickens with specific genetic backgrounds as earlobe color is breed-specific. The predominance of orange eyes in our study could be because eye color largely depends on the pigmentation (carotenoid pigments and blood supply) of a number of structures within the eye [25]. Accordingly, a prevalence of orange eye color was also observed in the study of Guni and Katule [23].
The predominant occurrence of pearl grey and pearl grey pied plumage colors concurred with the findings of Agbolosu et al [26], whereas bronze pied and brown pied plumage colors as reported by the same authors were barely observed in our study. The rare occurrence of white, brown, light grey, sky blue and bronze color types may be indicative of the threat faced by these varieties.
Main phenotypes had no effect on morphometric traits of female local guinea fowl in Tamale. In contrast, pearl grey pied male guinea fowl were inferior to their pearl grey and white counterparts with respect to body weight, body length and chest circumference. In addition, their wingspan was lower than that of pearl grey guinea fowl. Mean body weights were generally comparable to body weights reported for local guinea fowl in Ghana [15] and Nigeria [27]. Mean body lengths observed in our study were similar to body lengths reported for intensively raised local guinea fowl in Botswana [28]. In contrast, mean shank length and chest circumference of local guinea fowl in Tamale were lower than the values given by Ogah [27] and Tjetjoo et al [28], while mean wingspan measured in this study was higher than that reported by Ogah [27] for local Nigerian guinea fowl.
The occurrence of only brown beaks agreed with Ayorinde [29] who reported the same for Nigerian guinea fowl, while the predominance of purple helmet (54.6%) in this study contradicts other findings [26]. Furthermore, the latter study did not report brown/purplish brown head cap skin and white skin color as observed in our study. In contrast, Agbolosu et al [26] also reported a high share of dark skin color, which corresponds to our findings. According to Ayorinde [30], local guinea fowl have light yellow to white skins due to xanthophyll in feed and dark skin due to high melanin concentration.
We observed a number of color variants in local chickens in Tamale. Over half of the studied local chickens carried major genes; yet, occurrence of single major gene carriers and the respective allele frequencies were low. This indicates that these genes may soon disappear from local chicken populations. Although our study could not clearly confirm higher performances of major gene carriers as compared to the normal phenotype, they might be better adapted to local husbandry and climatic conditions. For guinea fowl, colors were more homogeneous. Two main phenotypes (pearl grey, pearl grey pied) and related genotypes were identified. This indicates a narrowing genetic diversity in local guinea fowl in Tamale.
The low contribution of major genes to local chicken resources and the low number of guinea fowl genotypes might be an indicator of low acceptance of related features by local producers. In designing community-based breeding programs, it is important to consider these trait preferences for both, local chickens and guinea fowl.
The present study provides basic information for broader genetic diversity studies to identify valuable poultry genetic resources and major genes for the development of breeding programs. Phenotypic variation of local chicken resources indicates a genetic diversity that may be worth conserving for future uses. It provides opportunity for selection and improvement of local chicken populations. Disproportionate distribution and low allele frequencies of major genes in local chickens as well as low genetic diversity in local guinea fowl emphasize that urgent action is needed to preserve valuable genes relevant to tropical production conditions. The utilization of well-adapted poultry genetic resources is the best route to conserving them for future uses; thus market-oriented production of local poultry breeds should be featured in agricultural policies in northern Ghana.
ACKNOWLEDGMENTS
This work was carried out as part of the UrbanFoodPlus Project, jointly funded by the German Federal Ministry of Education and Research (BMBF) and the German Federal Ministry for Economic Cooperation and Development (BMZ) under the initiative GlobE – Research for the Global Food Supply, grant number 031A242-A. Fieldwork of the first author was financially supported by a research grant from the German Institute for Tropical and Subtropical Agriculture (DITSL) in Witzenhausen. We would like to thank our research partners at the University for Development Studies, Tamale, Ghana, for providing enabling environment for the research. Last but not least, we are grateful to the participating poultry farmers for their willingness to participate in the study.
Figure 1
Occurrence (%) of major gene carriers in local chickens. Total n = 788, female chickens n = 652, male chickens n = 136. Some individuals were carriers of several major genes; therefore, percentages do not equal 100. Normal type: Individuals that carried none of the studied major genes. X2 statistics for differences between male and female chickens: 0.058 for naked neck, 0.232 for frizzle feather, 0.000 for silky feather, 0.628 for ptilopody, 1.277 for polydactyly; all p>0.05, 51.479 for crested head, 39.972 for rose comb, 15.474 for pea comb, 23.086 for normal type; all p<0.001.

Figure 2
Color distributions of local guinea fowl in individual household flocks. Total number of birds n = 394; total number of household flocks n = 35. B: black, Br: brown, G: grey, W: white, R: red, O: orange, Y: yellow, P: purple, Mu: multiple colors. X2 statistics: 252.7 for helmet color, 483.1 for head cap skin color, 141.8 for eye color, 142.7 for shank color, 293.9 for skin color; all p<0.001.
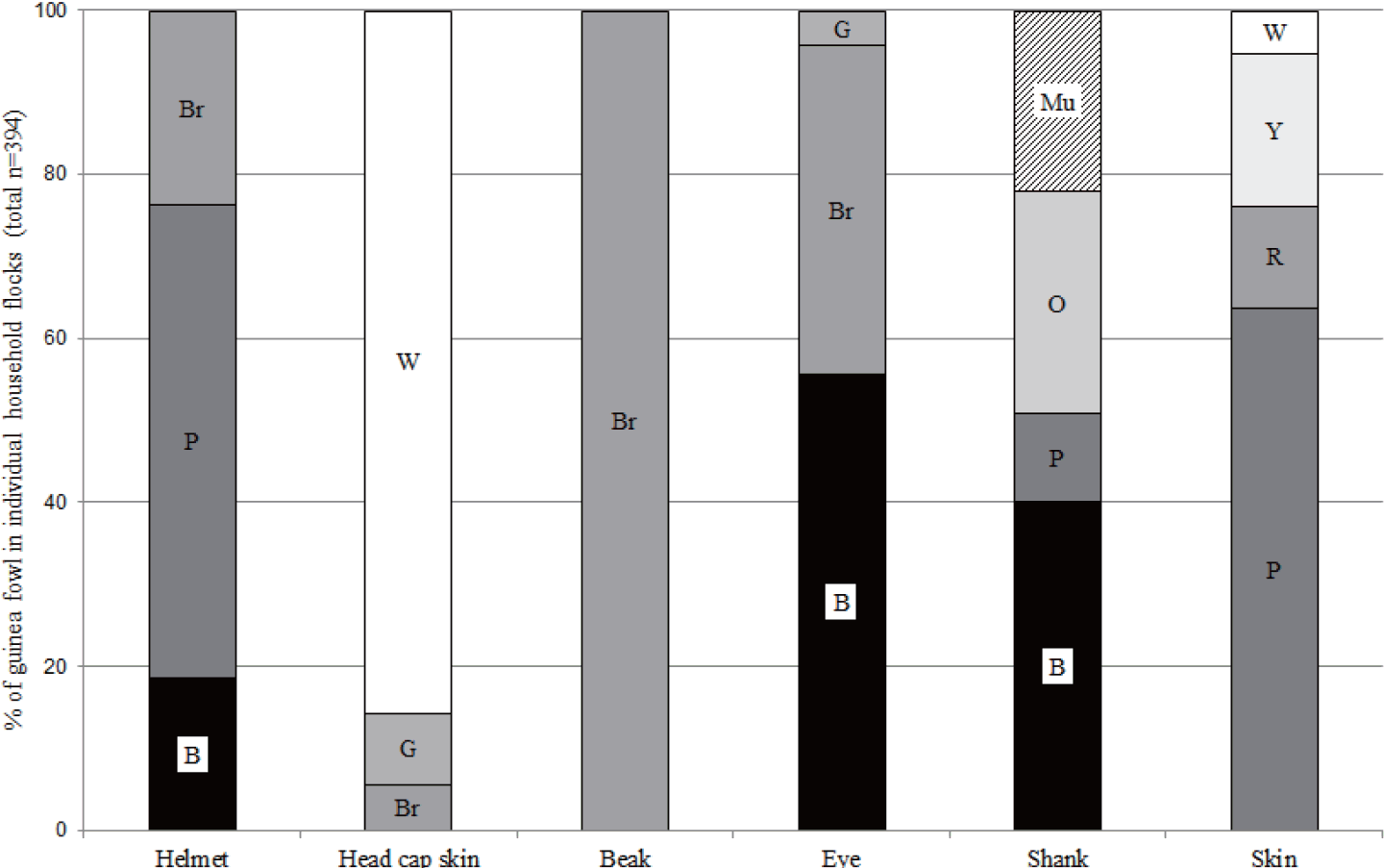
Table 1
Allele frequencies of major gene carriers in 788 local chickens
| Major gene | Number | Percentage | Allele frequency1) | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| Observed | Expected | Observed | Calculated | Expected | |
| Naked neck [Na] | 43 | 591 | 5.5 | 0.028** | 0.75 |
| Other2) [na+] | 745 | 197 | 94.5 | 0.972** | 0.25 |
| Frizzle [F] | 60 | 528 | 7.6 | 0.039** | 0.67 |
| Other [f+] | 728 | 260 | 92.4 | 0.961** | 0.33 |
| Silky [h] | 64 | 591 | 8.1 | 0.041** | 0.75 |
| Other [H+] | 724 | 197 | 91.9 | 0.959** | 0.25 |
| Polydactyly [Po] | 26 | 591 | 3.3 | 0.017** | 0.75 |
| Other [po+] | 762 | 260 | 96.7 | 0.983** | 0.25 |
| Ptilopody [Pti] | 3 | 591 | 0.4 | 0.002** | 0.75 |
| Other [pti+] | 785 | 197 | 0.96 | 0.998** | 0.25 |
| Crested head [Cr] | 237 | 591 | 30.1 | 0.164** | 0.75 |
| Other [cr+] | 551 | 197 | 69.9 | 0.836** | 0.25 |
| Rose comb [R] | 20 | 591 | 2.5 | 0.013** | 0.75 |
| Other [r+] | 768 | 197 | 97.5 | 0.987** | 0.25 |
| Pea comb [P] | 78 | 591 | 9.9 | 0.051** | 0.75 |
| Other [p+] | 710 | 197 | 90.1 | 0.949** | 0.25 |
Table 2
Color distributions of local chickens in individual household flocks
Table 3
Medians (means in parentheses) of morphometric traits measured in female local chicken phenotypes1)
| Traits | Naked neck (n = 27) | Frizzle feather (n = 27) | Silky feather (n = 23) | Crested head (n = 161) | Pea comb (n = 31) | Combinations2) (n = 94) | Normal type3) (n = 272) | Overall (n = 635) | SEM | x2 | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BW (kg) | 0.91ab (0.92) | 0.84ab (0.81) | 0.82ab (0.86) | 0.87ab (0.87) | 0.80ab (0.85) | 0.81b (0.82) | 0.87a (0.89) | 0.86 (0.87) | 0.01 | 20.01 | 0.003 |
| BL (cm) | 35.50 (35.28) | 35.90 (35.81) | 36.40 (36.01) | 36.10 (36.02) | 35.80 (35.99) | 35.90 (36.07) | 36.40 (36.23) | 36.20 (36.07) | 0.07 | 5.63 | 0.466 |
| CC (cm) | 23.40ab (23.01) | 22.30ab (22.31) | 22.10ab (22.36) | 23.40b (23.16) | 22.80ab (23.11) | 22.40b (22.31) | 23.40a (23.28) | 23.20 (23.01) | 0.07 | 28.75 | 0.000 |
| WS (cm) | 38.10 (38.06) | 40.00 (39.31) | 38.50 (38.97) | 38.70 (38.75) | 38.90 (39.05) | 39.05 (39.02) | 39.20 (39.09) | 39.00 (38.95) | 0.80 | 8.94 | 0.177 |
| SL (cm) | 9.00 (9.07) | 9.20 (9.11) | 9.20 (9.28) | 9.20 (9.13) | 9.10 (9.09) | 9.20 (9.24) | 9.20 (9.23) | 9.20 (9.18) | 0.19 | 6.27 | 0.394 |
Table 4
Medians (means in parentheses) of morphometric traits measured in male local chicken phenotypes1)
| Traits | Naked neck (n = 8) | Frizzle feather (n = 7) | Silky feather (n = 10) | Rose comb (n = 13) | Normal type2) (n = 86) | Overall (n = 124) | SEM | X2 | p-value |
|---|---|---|---|---|---|---|---|---|---|
| BW (kg) | 1.09 (1.03) | 0.95 (0.92) | 1.00 (1.01) | 1.06 (1.09) | 1.04 (1.01) | 1.04 (1.02) | 0.02 | 2.03 | 0.730 |
| BL (cm) | 38.55 (38.64) | 39.40 (38.89) | 38.40 (39.01) | 39.50 (39.03) | 38.80 (38.70) | 38.70 (38.77) | 0.20 | 0.52 | 0.972 |
| CC (cm) | 24.35 (23.86) | 24.20 (22.37) | 23.90 (24.14) | 24.80 (24.87) | 24.60 (24.33) | 24.40 (24.23) | 0.21 | 3.85 | 0.427 |
| WS (cm) | 43.20 (43.45) | 43.70 (43.16) | 42.85 (44.25) | 43.80 (43.78) | 43.65 (43.37) | 43.70 (43.48) | 0.24 | 0.88 | 0.928 |
| SL (cm) | 10.45 (10.71) | 10.80 (10.70) | 10.90 (10.96) | 11.20 (11.02) | 11.00 (10.88) | 11.00 (10.88) | 0.63 | 3.11 | 0.539 |
Table 5
Plumage color and related genotype of local guinea fowl in individual household flocks
| Plumage color | Genotype1) | Hens | Cocks | X2 | p-value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Flocks (n = 34) | Birds (n = 273) | Flocks (n = 32) | Birds (n = 121) | ||||
| Pearl grey pied (%) | M+/M+, I+/I+, D+/D+, W/w+ | 91.2 | 46.4 | 78.1 | 51.1 | 3.92 | 0.04 |
| Pearl grey (%) | M+/M+, I+/I+, D+/D+, w+/w+ | 79.4 | 42.5 | 71.9 | 41.0 | 1.87 | 0.17 |
| White (%) | −/−, −/−, −/−, W/W | 44.1 | 7.8 | 21.0 | 7.0 | 0.05 | 0.83 |
| Brown (%) | - | 14.7 | 1.2 | 3.1 | 0.3 | nd | - |
| Light grey (%) | M+/M+, i/i, D+/D+, w+/w+ | 8.8 | 0.9 | 3.1 | 0.5 | nd | - |
| Light/sky blue (%) | m/m, i/i, D+/D+, w+/w+ | 5.9 | 0.7 | - | - | nd | - |
| Bronze (%) | - | 2.9 | 0.4 | - | - | nd | - |
Table 6
Medians (means in parentheses) of morphometric traits measured in main female guinea fowl phenotypes1)
Table 7
Medians (means in parentheses) of morphometric traits measured in main male guinea fowl phenotypes1)
REFERENCES
1. FAO. The state of the world’s animal genetic resources for food and agriculture – in brief. Rome, Italy: FAO; 2007.
2. Okantah SA, Aboe PAT, Boa-Amponsem K, Dorward P, Bryant MJ. Small-scale chicken keeping in peri-urban Accra and Kumasi. Accra, Ghana: Animal Research Institute; 2003. A technical Report on the DFID (LPP) Project No. R7631.
3. Issaka BY, Yeboah RN. Socio-economic attributes of guinea fowl production in two districts in Northern Ghana. Afr J Agric Res 2016; 11:1209–17.

4. Mahrous M, Galal A, Fathi MM, Zein El-Dein A. Impact of naked neck (Na) and Frizzle (F) genes on growth performance and immunocompetence in chickens. Int J Poult Sci 2008; 7:45–54.

5. Fayeye TR, Ayorinde KL, Ojo V, Adesina OM. Frequency and influence of some major genes on body weight and body size parameters of Nigerian local chickens. Livest Res Rural Dev 2006; 18:Article #37
6. Dahloum L, Moula N, Halbouche M, Mignon-Grasteau S. Phenotypic characterisation of the indigenous chickens (Gallus gallus) in the northwest of Algeria. Arch Anim Breed 2016; 59:79–90.


7. Hagan JK, Adjei AI. Evaluation of the growth and carcass yield characteristics of crossbred naked-neck and frizzle cockerel phenotypes reared under hot and humid conditions. ARPN J Agric Biol Sci 2012; 7:576–82.
8. Osei-Amponsah R, Kayang BB, Naazie A. Phenotypic and genetic parameters for production traits of local chickens in Ghana. Anim Genet Res 2013; 53:45–50.

9. Government of Ghana. Northern Region [Internet]. 2015. [cited 2016 Jul 28]. Available from: http://www.ghana.gov.gh/index.php/about-ghana/regions/northern
10. Ghana Statistical Service. District Analytical Report: Tamale Metropolis [Internet]. 2014. [cited 2016 Jul 28] Available from: http://www.statsghana.gov.gh
11. Roessler R, Mpouam SE, Muchemwa T, Schlecht E. Classification of urban and peri-urban livestock farm types in Ouagadougou and Tamale. In : Tielkes E, editorManagement of land use systems for enhanced food security: conflicts, controversies and resolutions. Tropentag 2015; 2015 Sept 16–18; Hamburg, Germany.
12. FAO. Phenotypic characterisation of animal genetic resources. Rome, Italy: FAO Animal Production and Health Guidelines No. 11; 2012.
13. Cuesta ML. Pictorial guidance for phenotypic characterization of chickens and ducks. GCP/RAS/228/GER Working Paper No. 15. Rome, Italy: FAO; 2008.
14. Somes RG. Guinea fowl plumage colour inheritance, with particular attention on the dun colour. J Hered 1996; 87:138–42.


15. Yakubu A. Indigenous chicken flocks of Nasarawa State, Nigeria: their characteristics, husbandry and productivity. Trop Subtrop Agroecosyst 2010; 12:69–76.
16. DAD-IS (Domestic Animal Diversity Information System) [Internet]. 2016. [cited 2016 Jul 20]. Available from: http://dad.fao.org/
17. Wright D, Boije H, Meadows JRS, et al. Copy number variation in Intron 1 of SOX5 causes the pea-comb phenotype in chickens. PLoS Genet 2009; 5:e1000512



18. Hagan JK, Adomako K, Olympio OS. Effects of naked neck and frizzle genes on growth performance and carcass characteristics of crossbred cockerels. J Sci Technol 2011; 31:42–7.

19. Oguntunji AO, Ayandiji A, Kehinde AL. Awareness on genetic ‘erosion’ of some economic genes in Nigerian local chicken. Afr J Livest Ext 2007; 5:32–6.
20. Baeza E, Williams J, Guemene D, Duclos M. Sexual dimorphism for growth in Muscovy ducks and changes in insulin-like growth factor I (IGF-I), growth hormone (GH) and triiodothyronine (T3) plasma levels. Reprod Nutr Dev 2001; 41:173–9.


21. Egahi JO, Dim NI, Momoh OM, Gwaza DS. Variations in qualitative traits in the Nigerian local chicken. Int J Poult Sci 2010; 9:978–9.

22. Youssao IAK, Tobada PC, Koutinhouin BJ, et al. Phenotypic characterisation and molecular polymorphism of indigenous poultry populations of the species Gallus gallus of Savannah and Forest ecotypes of Benin. Afr J Biotechnol 2010; 9:369–81.
23. Guni FS, Katule AM. Characterisation of local chickens in selected districts of the southern highlands of Tanzania: I. Qualitative characters. Livest Res Rural Dev 2013; 25:Article #153
24. Negassa D, Melesse A, Banerjee S. Phenotypic characterisation of indigenous chicken populations in South-eastern Oromia Regional State of Ethiopia. Anim Genet Res 2014; 55:101–13.

25. Crawford RD. Poultry breeding and genetics. Amsterdam The Netherlands: Elsevier; 2013. Cited in: Guni FS, Katule AM. Characterisation of local chickens in selected districts of the Southern highlands of Tanzania: I. Qualitative characters. Livest Res Rural Dev 1990;25: Article #153
26. Agbolosu AA, Ahunu BK, Aboagye GS, Naazie A, Kayang BB. Variation in some qualitative traits of the indigenous guinea fowl in Northern Ghana. Global J Anim Sci Res 2015; 3:30–5.
27. Ogah DM. Variability in body shape characters in an indigenous guinea fowl (Numida meleagris L.). Slovak J Anim Sci 2013; 46:110–4.
28. Tjetjoo SU, Moreki JC, Nsoso SJ, Madibela OR. Growth performance of guinea fowl fed diets containing yellow maize, millet and white sorghum as energy sources and raised under intensive system. Pak J Nutr 2013; 12:306–12.

29. Ayorinde KL. External characteristics of four indigenous helmeted guinea fowl varieties (Numidia meleagris Galeata Pallas) in Nigeria. Nigerian J Anim Prod 1989; 16:47–54.
30. Ayorinde KL. The spice of life. Amali SOO, editorThe 71st inaugural lecture. Ilorin, Nigeria: Library and Publications Committee, University of Ilorin; 2004.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





