 |
 |
| Anim Biosci > Volume 29(9); 2016 > Article |
|
Abstract
Rennet, a complex of enzymes found in the stomachs of ruminants, is an important component for cheese production. In our study, we described that yak chymosin gene recombinant Pichia pastoris strain could serve as a novel source for rennet production. Yaks total RNA was extracted from the abomasum of an unweaned yak. The yak preprochymosin, prochymosin, and chymosin genes from total RNA were isolated using gene specific primers based on cattle chymosin gene sequence respectively and analyzed their expression pattern byreal time-polymerase chain reaction. The result showed that the chymosin gene expression level of the sucking yaks was 11.45 times higher than one of adult yaks and yak chymosin belongs to Bovidae family in phylogenetic analysis. To express each, the preprochymosin, prochymosin, and chymosin genes were ligated into the expression vector pPICZ╬▒A, respectively, and were expressed in Pichia pastoris X33. The results showed that all the recombinant clones of P. pastoris containing the preprochymosin, prochymosin or chymosin genes could produce the active form of recombinant chymosin into the culture supernatant. Heterologous expressed prochymosin (14.55 Soxhlet unit/mL) had the highest enzyme activity of the three expressed chymosin enzymes. Therefore, we suggest that the yak chymosin gene recombinant Pichia pastoris strain could provide an alternative source of rennet production.
Chymosin, previously called rennin, is an aspartate proteinase which is found in the fourth stomach of suckling calves, among other animals (Ustunol and Hicks, 1990). This enzyme can act against the Phe105-Met106 peptide bond which is present in milk k-casein molecules (Foltmann, 1969), generating insoluble para-k-casein and resultant clotting of milk (McMahon et al., 1984). It is traditionally used in the precursor step during the production of different types of cheeses (Rao et al., 1998). Chymosin is synthesized as a precursor protein (preprochymosin, 381 amino acids) which has a 16-amino acid signal peptide. This signal sequence is removed to produce prochymosin. This zymogen is then converted to active chymosin by cleavage of a 42-amino acid pro-peptide at the NH2-terminus facilitated by the acidic conditions found in the stomach (Pedersen et al., 1979).
To date, commercial rennet comes from four different sources: Animal, plant, microbial, and recombinant rennet. All four types of commercial rennet are used by the cheese industry (Vallejo et al., 2008). Its critical importance in the production of cheeses combined with the current high demand for cheeses has resulted in a worldwide shortage of rennin. This shortage is made more serious due to a contraction of the veal calf market; bovine rennet has traditionally been extracted from the abomasum of unweaned calves. This huge demand has led to several substitutes, notably plant, micriobial and recombinant rennet. Plant-derived rennet has been extracted from plants like the artichoke (Chazarra et al., 2007), however, these plants are only found in restricted geographic areas and used in the production of certain specific types of cheese. Microbial rennet has been obtained from the culture supernatants of microbes such as Aspergillus oryzae (Vishwanatha et al., 2010) or Endothia parasitica (Sardinas, 1968). This type of rennet contains low levels of specific proteases which may induce bitter flavors in the products and engender low curd yields during the cheese making process (Davies and Law, 1984). The remaining type of rennet commonly used, recombinant rennet, has been obtained from recombinant microorganisms containing the chymosin gene from Bos Taurus (Emtage et al., 1983; Cullen et al., 1987) has and features of both a low cost and a stable source.
The yak (Bos grunniens) is an important grazing livestock found on the Tibetan plateau where few other domestic animals can survive. It provides most of the animal products (meat, fur, milk, etc.) for people who live on Tibetan plateau (He and Li, 2004). Currently, goat chymosin (Vega-Hernandez et al., 2004), camel chymosin (Kappeler et al., 2006) and buffalo chymosin (Vallejo et al., 2008) have been produced by microorganisms. However, the chymosin of yak owing to high commercial value has not been reported previously. P. pastoris is an excellent eukaryotic expression system yielding a high-level of expression of recombinant proteins, protein processing, protein folding, and posttranslational modification. In addition, P. pastoris has obtained the ratification of U.S. Food and Drug Administration (FDA) (Zhang et al., 2009) as a safe and effective expression system. Therefore, P. pastoris is the favored choice for use as the host to express recombinant yak chymosin. In this paper we report the cloning, bioinformatic analysis, expression detection and enzymatic analysis of heterologous yak chymosin, and provide a novel chymosin for use in cheese production.
This study was approved by the Southwest University for Nationalities Institutional Animal Care and Use Committee (permit number: 2013-3-2).
Three 2-months old and three 3 to 4 years old healthy male yaks from the SongPan county of Sichuan province, China were selected for this experiment. Tissue samples from abomasum tissue were collected after animals were euthanized.
Escherichia coli DH5╬▒ cells and pMD19-T vector (TAKARA, Dalian, China) were used for DNA manipulation and amplification. P. pastoris X33 and pPICZ╬▒A were used for expression of the preprochymosin, prochymosin, and chymosin genes.
Luria Bertani medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl, at a pH of 7 to 7.5) supplemented with ampicillin (100 ╬╝g/mL) or Zeocin (25 ╬╝g/mL) was used for the cultivation of the E. coli DH5╬▒. Yease extract, peptone, dextrose (YPD) medium (10 g/L yeast extract, 10g/L peptone, and 20 g/L glucose) supplemented with Zeocin (100 ╬╝g/mL) was used for the selection of the transformant P. pastoris X33. Buffered glycerol complex (BMGY) (20 g/L tryptone, 10 g/L yeast extract, 3 g/L K2HPO4, 11.8 g/L KH2PO4, 100 mL/L 10├Śyeast nitrogen base without amino acids (YNB), 1 mL/L 500├ŚBiotin and 10 mL/L glycerin) and buffered methanol-complex (BMMY) (20 g/L tryptone, 10 g/L yeast extract, 3 g/L K2HPO4, 11.8 g/L KH2PO4, 100 mL/L 10├ŚYNB, 1 mL/L 500├Śbiotin and 5 mL/L Methanol) were used for the expression of recombinant P. pastoris.
Tissue samples of abomasum were collected immediately following euthanasia from a sucking yak. The tissue was rinsed in diethyl pyrocarbonate (DEPC)-treated water and then snap frozen in liquid nitrogen. Total RNA was isolated from stomach using Trizol reagent (Invitrogen, Grand Island, NY, USA), following the manufacturerŌĆÖs instructions. The quality and concentration of each RNA sample was assessed by ultraviolet spectrophotometry.
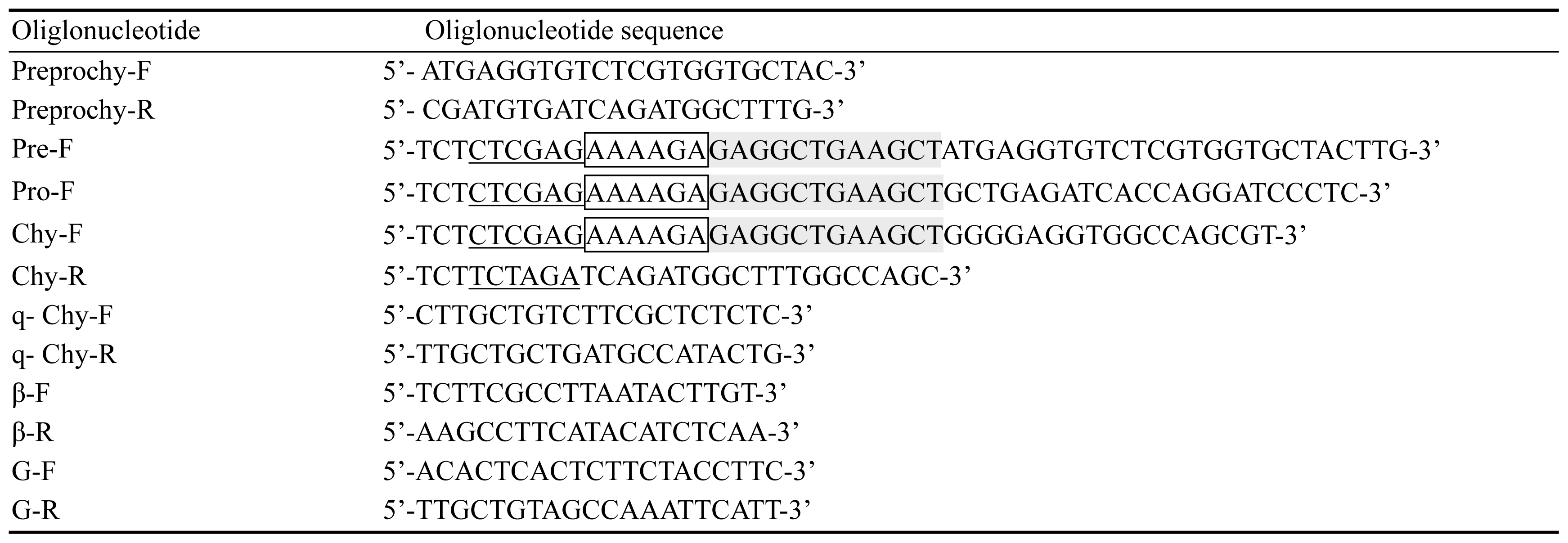
The first strand cDNA was synthesized by using PrimeScript RT-PCR Kit (TAKARA, Dalian, China), following the instructions recommended by the manufacturer. The gene of preprochymosin was obtained by polymerase chain reaction (PCR) using sequence specific primers Preprochy-F and Preprochy-R (Table 1) which were designed from the B. Taurus preprochymosin sequence (Genebank accession code: NM_1890994). The reaction mixture contained cDNA template 1 ╬╝L, 10├ŚEx Taq Buffer 2.5 ╬╝L, dNTP Mix 2 ╬╝L, Preprochy-F 1 ╬╝L, Preprochy-R 1 ╬╝L, ExTaq DNA polymerase 0.13 ╬╝L, Mgcl2 2 ╬╝L and ddH2O 15.37 ╬╝L. The program for this PCR reaction was 95┬░C for 2 min, 35 cycles of 95┬░C for 30 s; 66┬░C for 1 min and 72┬░C for 2 min; and a final step of 72┬░C for 5 min. The PCR production was then cloned into the pMD19-T vector (TAKARA, Dalian, China), denominated as pMD19-T-Preprochy, and the inserted gene fragment was sequenced by Sangon Biotech Co., Ltd (Shanghai, China). The preprochymosin, prochymosin, and chymosin genes were amplified from pMD19-T-Preprochy using gene specific primers: Pre-F and Chy-R, Pro-F and Chy-R, Chy-F and Chy-R (Table 1), and then the amplified fragments were inserted into pMD19-T vector, denominated as pMD19-T-Pre, pMD19-T-Pro, and pMD19-T-Chy respectively.
Three XhoI and XbaI fragments of preprochymosin, prochymosin, and chymosin were obtained from the pMD19-T vector containing the same cDNA sequences and were cloned into the pPICZ╬▒A vector previously digested with the same restriction enzyme. The vectors were then transformed into E. coli DH5╬▒. The recombinant pPICZ╬▒A plasmid was linearized by SacI then transformed into P. pastoris X33 by electroporation. Finally, the transformed cells were screened on yeast extract peptone detrose medium with sorbitol (YPDS) (1% [w/v] yeast extract, 2% [w/v] peptone, 2% [w/v] dextrose, 1 M sorbitol, and 2% [w/v] agar) plates containing zeocin at a final concentration of 100 ╬╝g/mL. Integration of the preprochymosin, prochymosin, and chymosin genes into the P. pastoris genome was confirmed by PCR using yeast genomic DNA as a template and the gene specific primers in Table 1.
The recombinant colonies of P. pastoris were cultured in 25 mL of BMGY at 30┬░C and spun at 240 rpm until culture reached an OD600 = 2 to 6 (~16 to 18 hours). The cells were harvested by centrifuging at 1,500├Śg for 5 minutes at room temperature. Then, supernatant was decanted and the cell pellet resuspended to an OD600 of 1.0 in BMMY medium to induce expression (approximately 100 to 200 mL). The culture was placed in a 500 mL flask. Next, the flask was covered with 7 layers of sterile gauze and returned to incubator to continue growth of the cells. Pure methanol was added to the culture to a final concentration of 1% every 24 hours to maintain induction. One mL of the expression culture supernatant was transferred to a 1.5 mL microcentrifuge tube every 24 hours. These samples were used to analyze enzyme activity and detect the optimal time of the harvest. Saturated ammonium sulfate was added to the supernatant to a final concentration of 60%. The supernatant was then centrifuged at 12,000├Śg for 2 min and the supernatant protein was collected for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
The induced culture of the recombinant P. pastoris was centrifuged at 12,000├Śg for 2 min. Then the supernatant was directly used for the determination of chymosin activity.
Chymosin activity in the culture supernatant was measured by the method of Arima K (Arima et al., 1970). Briefly, 0.5 mL of enzyme solution was placed into a 10 mL test tube followed by the addition of 5 mL of 10% (w/w) solution of skim-milk powder containing 0.01 M calcium chloride (All reagents are preincubated at 35┬░C for at least 10 minutes). The time (t) elapsing between the mixing of reagents and the first appearance of solid material was recorded. Under the above conditions, the amount of enzyme sufficient to clot the milk solution in 40 min is defined as a Soxhlet unit (SU).
All detections were repeated 3 times and the results are expressed as the average of the three replicates.
The expression of the yak chymosin gene was detected in abomasums tissue from different ages of yaks using the real-time PCR method. The total RNA of abomasums was extracted from abomasums tissue of three sucking yaks and three adult yaks. The first strand cDNA was synthesized by the same method described in the previous section. All cDNA samples were used as the templates in Real-time PCR analysis. The reaction system was as follows: Template 2 ╬╝L, SsoFast EvaGreen supermix (BIO-RAD, Hercules, CA, USA) 5 ╬╝L, gene-specific primers q-Chy-F 0.5 ╬╝L, gene-specific primers q-Chy-R 0.5 ╬╝L (Table 1), ddH2O 2 ╬╝L, in a total volume of 10 ╬╝L. Real-time PCR was performed with the following program: 50┬░C for 2 min, 95┬░C for 10 min, 40 cycles of 95┬░C for 15 s; 57.6┬░C for 1 min; then 95┬░C for 15 s, 65┬░C for 15 s. The gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ╬▓-actin were used as loading controls in this experiment (GAPDH primer G-F, GAPDH primer G-R, ╬▓-actin primer ╬▓-F and ╬▓-actin primer ╬▓-R were present in Table 1).
The cDNA sequence of the chymosin gene was searched against the non-redundant (nr) database at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/USA) using the Blastx algorithm. The signal peptide analysis was conducted by the online tool of the SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/). To clarify the relationships between yak and chymosin from other species, amino acid sequences from yak chymosin and 53 other chymosins were retrieved from GenBank, aligned and the phylogenetic tree was created by Mega version 5.0 utilizing the maximum likelihood and neighbor-joining approach using Tamura-Nei evolutionary model (Tamura et al., 2011). Bootstrap analysis with 1,000 replicas was used.
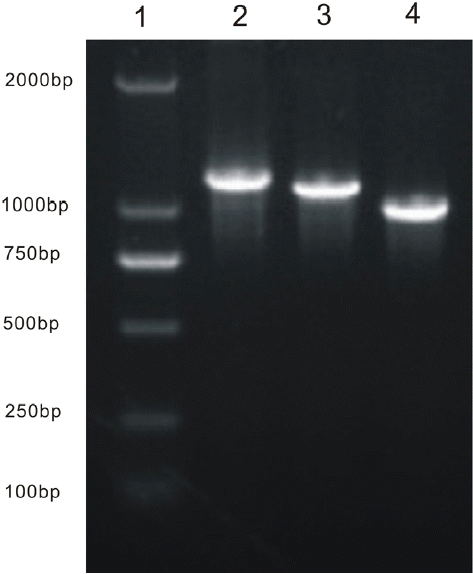
Using RT-PCR and the gene-specific primer pairs Pre-F and Chy-R, Pro-F and Chy-R, and Chy-F and Chy-R (Table 1), three bands of sizes 1,182, 1,135, and 1,008 bp were obtained (Figure 1) corresponding to the preprochymosin, prochymosin, and chymosin genes, respectively. The three fragments were cloned into a pMD19-T vector, where the gene was sequenced and deposited in GenBank (Accession no. JX839990).
The Yak chymosin gene has a 1,146 bp open reading frame and encodes 381 amino acids which also contains a 16-amino acid signal peptide that is removed to generate prochymosin from preprochymosin. This zymogen will become chymosin by cleavage of the 42-amino acid propeptide at the NH2-terminus.
Alignment of the DNA sequences showed a sequence identity of yak chymosin (JX839990.1) with chymosin from Bos Taurus (NP851337.1, 99.39%), Bubalus bubalis (XP006065015.1, 98.17%), Ovis aries (NP001009804.1, 95.55%), Capra hircus (NP001272688.1, 95.38%), Camelus dromedarius (NP_001290503.1, 88.13%), and Rattus norvegicus (NP_064476.1, 77.66%). Amino acids sequence alignment of Yak chymosin showed the similarity between yaks and Bos Taurus, Bubalus bubalis, Ovis aries, Capra hircus, Camelus dromedarius, and Rattus norvegicus was 99.21%, 98.16%, 93.96%, 93.70%, 83.73%, 72.18%, respectively.
A phylogenetic analysis of different chymosin from yak and 53 other animals was constructed based on their amino acid sequences (Figure 2). The results showed that there were four big groups and 2 small groups in this phylogenetic tree. Group I included Bovidae, Camelidae and Cetacea. Group II included Vespertilionidae, Felidae, Mustelidae, Canidae, Ursidae, and so on. Group III included Primates, Erinaceinae, Leporidae, Chrysochloridae, Elephantidae. Group IV included Muridae and Lemuridae formed Group V. Sarcophilus harrisii and Monodelphis domestica formed Group VI. The yak chymosin was placed in the Group I, and Bos Taurus, Bison bison, Bos mutus, Bos grunniens, and Bubalus bubalis form a sub group, thus suggesting a closel evolutionary relationship among them. Interestingly, Bos mutus (wild yak) and Bos grunniens (domestic yak) did not form a close group, although researchers considered that they belong to different subspecies of yak.
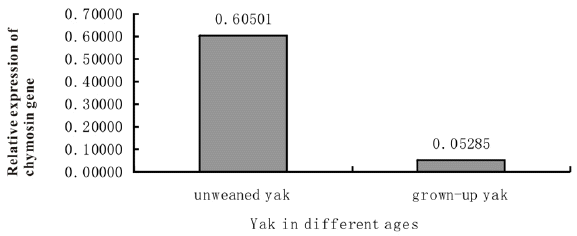
To characterize the change of chymosin gene expression between the sucking yak and adult yak, the expression level of this gene in three sucking yaks and three adult yaks was detected by qPCR (real time quantitative PCR). The results showed that the expression level of this gene in abomasum tissue from the sucking yaks was 11.45 time higher than in adult yaks. This observation suggests that the yak chymosin gene plays an important role in sucking yak to digest milk components (Figure 3).
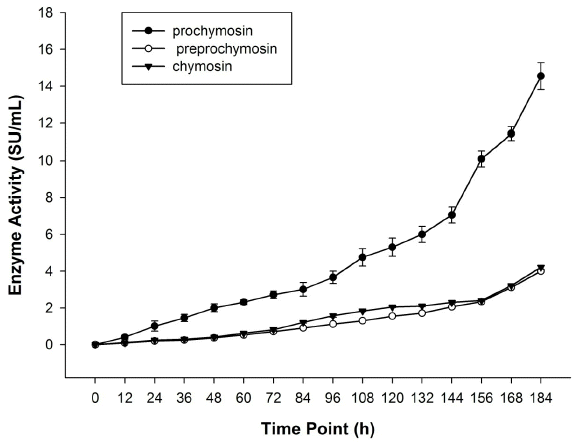
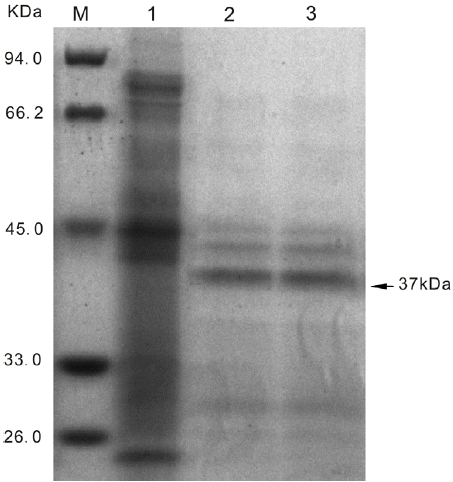
Preprochymosin, prochymosin, and chymosin were expressed using P. pastoris X33 and expression vector pPICZ╬▒A. All the clones containing preprochymosin, prochymosin, or chymosin gene exhibited active chymosin in the supernatant, without the need of an in vitro activation process (Figure 4). The chymosin activity of the culture supernatant was 4.00 SU/mL, 14.55 SU/mL, and 4.21 SU/mL corresponding to the preprochymosin, prochymosin, and chymosin respectively after 184 h of incubation (Figure 5). But, only the culture supernatant which expressed the prochymosin sequence showed a clearly heterologous protein band when detected by SDS-PAGE method (Figure 6).
The traditional sources of rennet are not sufficient to meet current demands and the cost of rennet production is high due to a global shortage of calves (Yuan et al., 2010). Furthermore, although researchers have made steady progress to expand the sources and increase the activity of rennet with some success, there are still major obstacles to overcome including the continued low supply and high production costs of rennet. More research is needed to solve these problems. With the development of genetic engineering technology, is now possible to use recombinant DNA technology and micro-organisms to produce recombinant rennet to fulfill the shortage of rennet. In our study, P. pastoris was chosen as a host to express the yak chymosin gene and ultimately provide a novel source of rennet for cheese production.
Rennet can be divided into three types: prochymosin A, B, and C. The 244th amino acid residue of A type is Asp, whereas in the B type it is Gly. Differences in activity between type A and B can be attributed to the strong electron activity of Asp244 found in type A which has been shown to enhance its binding affinity. Prochymosin A has a stronger activity to k-casein, but less is stable than the prochymosin B. Prochymosin C is an autolysate of prochymosin A, the Asp286-Glu287-Phe288 of A is removed to generate Prochymosin C (Kageyama, 2002). Yak chymosin, in which the 244th amino acid residue is Asp, belongs to the type A and therefore has a strong activity against k-casein.
In the present paper the preprochymosin, prochymosin, and chymosin genes were cloned from RNA which was extracted from the abomasum of a suckling yak using RT-PCR techniques. The cDNA sequences obtained (Genbank accession code: JX839990) showed a high homology with its Bos taurus counterparts. The cDNA sequences were inserted into pPICZ╬▒A vector and expressed in P. pastoris X33. All the clones led to the expression of the active chymosin form. However, we only get a strong heterologous protein band using the culture supernatant of P. pastoris containing the prochymosin gene by SDS-PAGE. It can be deduced that the other two recombinants of P. pastoris have a low expression level. The detected enzyme activity of the recombinant chymosin was due to the Lys-Arg sequence and Glu-Ala repeats which were designed in the primer. The ╬▒-factor signal sequence was removed by a signal peptidase system to produce active chymosin, which involved the action of an endopeptidase encoded by the Kexin (KEX2) gene to cleave the Lys-Arg sequence and a dipeptidyl aminopeptidase encoded by the STE13 gene to remove the Glu-Ala repeats (Brake et al., 1984). In the case of the cloned yak prochymosin and preprochymosin, these recombinants also showed rennet activity that could be explained by the presence of the target Lys-Argin. The endopeptidase coded by KEX2 gene could cleave this target to generate the active protein in the supernatant which is similar to that named as pseudo chymosin by Pedersen and Foltmann (1973). When buffalo chymosin, prochymosin, and preprochymosin genes were expressed in Pichia pastoris GS115, only the prochymosin was shown to have enzymatic activity (Vallejo et al., 2008). This discrepancy may result from using a different expression vector and/or different source of rennet genes.
In this work, we successfully expressed the yak chymosin, prochymosin, and preprochymosin genes in P. pastoris and obtained their active form in the culture supernatants. Because the heterologous chymosin protein does not need activation and can be secreted into culture supernatant, we do not need to lyse the cells to purify the rennet. This attribute can reduce the number of production steps necessary and ultimately lower costs. Additionally, the obtained yak chymosin doesnŌĆÖt have an extra sequence which has no effect on its activity compared to prochymosin from buffalo (Vallejo et al., 2008), which can further reduce the cost to produce it. Therefore we think that the recombinant strains obtained in this study could be an excellent candidate for the production of rennet.
Finally, in this study we cloned the preprochymosin, prochymosin, and chymosin gene and expressed the genes in Pichia pastoris. Yak chymosin gene had a 1,146 bp coding sequence which encodes a 381 amino acid long preprochymosin. Prochymosin can be generated from preprochymosin by cleaving off a 16-amino acid signal peptide and then removing 42-amino acid propeptide from its N-terminal produce chymosin. The Phylogenetic analysis clearly indicated that yak chymosin gene belongs to Bovidae family and was highly expressed in unweaned yak.
All heterologous expressed yak preprochymosin, prochymosin, and chymosin exhibit chymosin activity and cause milk-clotting, but the greatest expression in this study was from the heterologous expressed prochymosin gene (14.55 SU/mL). Therefore, the heterologous expressed prochymosin from our recombinant Pichia pastoris strain can be used as milk-clotting enzymes in cheese production.
ACKNOWLEDGMENTS
This work was supported by a grant from Sichuan Province Youth Science and technology innovation team project (2015TD0025), The Fundamental Research Funds for the central Universities (2014NZYQN59), and Innovation team project for conservation and utilization of yak genetic resources (13CXTD01).
Figure┬Ā1
Polymerase chain reaction products of yak preprochymosin, prochymosin, and chymosin gene. Lane 1: DNA marker DL2000; Lane 2: preprochymosin gene; Lane 3: prochymosin gene; Lane 4: chymosin gene.
Figure┬Ā2
The phylogenetic tree of chymosinamino sequence (constructed by Mega version 5.0 software).

Figure┬Ā4
Milk-clotting test of recombinant preprochymosin, prochymosin, and chymosin expressed by P. pastoris. 1: milk treated with the culture supernatant with the blank pPICZ╬▒A vector; 2: milk treated with the culture supernatant of containing recombinant preprochymosin; 3: milk treated with the culture supernatant with recombineant prochymosin; 4: milk treated with the culture supernatant with recombinant chymosin.

Figure┬Ā5
Activity curves of the recombinant preprochymosin, prochymosin, and chymosin expressed in P. pastoris which was induced during 184 h.
Figure┬Ā6
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of recombinant P. pastoris supernatants. Lane M: protein marker; Lane 1: negative control (blank pPICZ╬▒A vector without insert); Lane 2 and lane 3: pPICZ╬▒A vector with prochymosin gene.
REFERENCES
Arima K, Yu J, Iwasaki S. 1970. Milk-clotting enzyme from mucor pusillus var. Lindt Methods Enzymol 19:446ŌĆō459.

Brake AJ, Merryweather JP, Coit DG, Heberlein UA, Masiarz FR, Mullenbach GT, Urdea MS, Valenzuela P, Barr PJ. 1984. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 81:4642ŌĆō4646.



Chazarra S, Sidrach L, Lopez-Molina D, Rodriguez-Lopez JN. 2007. Characterization of the milk-clotting properties of extracts from artichoke (Cynara scolymus, L.) flowers. Int Dairy J 17:1393ŌĆō1400.

Cullen D, Gary GL, Wilson LJ, Hayenga KJ, Lamsa MH, Rey MW, Norton S, Berka RM. 1987. Controlled expression and secretion of bovine chymosin in Aspergillus nidulans. Nat Biotechnol 5:369ŌĆō376.

Davies FL, Law BA. 1984. Advances in the Microbiology and Biochemistry of Cheese and Fermented Milk. Elsevier Applied Science Publishers; London, UK:
Emtage JS, Angal S, Doel MT, Harris TJ, Jenkins B, Lilley G, Lowe PA. 1983. Synthesis of calf prochymosin (prorennin) in Escherichia coli. Proc Natl Acad Sci USA 80:3671ŌĆō3675.



He AX, Li L. 2004. Study on the relation between yak performance and ecological protection. In : Proceedings of the Fourth International Congress on Yak; Chengdu, China.
Kageyama T. 2002. Pepsinogens, progastricsins, and prochymosins: structure, function, evolution, and development. Cell Mol Life Sci 59:288ŌĆō306.



Kappeler SR, van den Brink HM, Rahbek-Nielsen H, Farah Z, Puhan Z, Hansen EB, Johansen E. 2006. Characterization of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk. Biochem Biophys Res Commun 342:647ŌĆō654.


McMahon DJ, Brown RJ, Ernstrom CA. 1984. Enzymic coagulation of milk casein micelles. J Dairy Sci 67:745ŌĆō748.

Pedersen VB, Christensen KA, Foltmann B. 1979. Investigations on the activation of bovine prochymosin. Eur J Biochem 94:573ŌĆō580.


Pedersen VB, Foltmann B. 1973. The amino acid sequence of the first 61 residues of chymosin (rennin ec 3.4.4.3). FEBS Lett 35:250ŌĆō254.


Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. 1998. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597ŌĆō635.



Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. Mega5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731ŌĆō2739.



Vallejo JA, Ageitos JM, Poza M, Villa TG. 2008. Cloning and expression of buffalo active chymosin in pichia pastoris. J Agric Food Chem 56:10606ŌĆō10610.


Vega-Hernandez MC, Gomez-Coello A, Villar J, Claverie-Martin F. 2004. Molecular cloning and expression in yeast of caprine prochymosin. J Biotechnol 114:69ŌĆō79.


Vishwanatha KS, Appu Rao AG, Singh SA. 2010. Production and characterization of a milk-clotting enzyme from Aspergillus oryzae MTCC 5341. Appl Microbiol Biotechnol 85:1849ŌĆō1859.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





