 |
 |
|
|
Abstract
Ex vivo digestion of proteins and fat in Red Chittagong Cattle milk from Bangladesh was carried out using human gastrointestinal enzymes. This was done to investigate the protein digestion in this bovine breed’s milk with an especial focus on the degradation of the allergenic milk proteins; αs1-casein and β-lactoglobulin and also to record the generation of peptides. Lipolysis of the milk fat and release of fatty acids were also under consideration. After 40 min of gastric digestion, all the αs-caseins were digested completely while β-lactoglobulin remained intact. During 120 min of duodenal digestion β-lactoglobulin was reduced, however, still some intact β-lactoglobulin was observed. The highest number of peptides was identified from β-casein and almost all the peptides from κ-casein and β-lactoglobulin were identified from the gastric and duodenal samples, respectively. No lipolysis was observed in the gastric phase of digestion. After 120 min of duodenal digestion, milk fat showed 48% lipolysis. Medium (C10:0 to C16:0) and long (≥C17:0) chain fatty acids showed 6% to 19% less lipolysis than the short (C6:0 to C8:0) chain fatty acids. Among the unsaturated fatty acids C18:1∑others showed highest lipolysis (81%) which was more than three times of C18:2∑all and all other unsaturated fatty acids showed lipolysis ranging from 32% to 38%. The overall digestion of Bangladeshi Red Cattle milk was more or less similar to the digestion of Nordic bovine milk (Norwegian Red Cattle).
The use of ex vivo digestion model is important for understanding the mechanisms involved in food digestion and to mimic the human digestion. There are some other purposes as well for using such models are - e.g. investigation of bioactive components, study the survivability of drugs through the gastrointestinal (GI) tract, investigation of the digestibility of food allergens, to design food-based delivery system in the GI tract and to study the structural changes of ingested components.
The most challenging physiological parameters are the individual variation in enzymes, acid and bile salt secretion, substrate availability and retention time in the gastric and intestine. The commercial enzyme(s) preparations are purified from different animal species. Generally, the same enzymes purified from different animal species are likely to vary in specificity, functional enzymatic parameters and stability (Furlund et al., 2013). But, to date, most of the in vitro studies regarding protein degradation and peptide generation have been done by using the commercial proteases, mainly of porcine or bovine origin. Whereas, human GI juices are a complex mixture of enzymes, with their isoforms and inhibitors, and bile salts. The sample characteristics, enzyme activity, ionic composition, used mechanical stresses and the digestion duration profoundly affect the results of in vitro digestion (Hur et al., 2011) and simulation of in vivo condition will never be complete. However, making the compromise between accuracy and ease of utilization, in vitro model digestion could be used as a rapid screening tool for foods with different composition and structures (Hur et al., 2011). Few suggest the use of single enzymes in in vitro model digestion but the use of a mixture of enzymes is more realistic and some workers prefer to use human digestive juices.
Milk protein digestion and peptides from in vitro digested milk has been studied by several authors (Almaas et al., 2006; Inglingstad et al., 2010; Almaas et al., 2011; Furlund et al., 2013; Devle et al., 2014; Tidona et al., 2014; Islam et al., 2014b). But still more knowledge is needed because of high variability in milk composition between breeds (Miranda et al., 2004; Abd El-Salam and El-Shibiny 2011; Medhammar et al., 2012; Islam et al., 2014a) and in in vitro digestion protocol used (Hur et al., 2011; Kopf-Bolanz et al., 2012; Furlund et al., 2013; Islam et al., 2014b). The structure of milk and milk protein composition may have an influence on its digestibility (Almaas et al., 2006). Tidona et al. (2014) found more rapid degradation of β-lactoglobulin (β-Lg)-I when β-Lg-II is absent in donkey’s milk. The heterogeneity in the amino acid composition of milk may result in variation in peptide formation and content after proteolysis (Ulleberg, 2011). The rapid degradation pattern of allergenic milk proteins, β-Lg and αs1-casein, in Bangladeshi buffalo milk (Islam et al., 2014; unpublished data) also increased interest in checking other bovine milk from Bangladesh. In a previous study, Islam et al. (2014a) concluded that buffalo and Red Chittagong Cattle (RCC) milk showed the highest compositional characteristics for nutritional and technological properties. The dairy potentialities, disease resistance, tolerance to harsh environmental conditions along with low input supply makes the RCC a potential interesting dairy genetic resource. Accordingly, several studies have been conducted regarding the management of the RCC, its productive and reproductive performances, phenotypes and genotypes and recently on principal milk components but studies have yet to test the nutritional quality of the milk.
Very few studies have been conducted regarding the milk lipid digestion and according to Miled et al. (2000), in general, few studies have been reported on lipid digestion. Lipid digestion is more complex than protein digestion regarding the enzymes and physiological conditions in the gut. Factors like food matrix and buffering capacity, emulsion type (oil based/water based), individual secretion of both lipolytic enzymes and bile salts affect the hydrolysis of dietary lipids. The digestion of one nutrient may affect the digestion of others. Devle et al. (2014) and Islam et al. (2014; unpublished data) showed different effects of milk lipids on the protein digestion in cow (Norwegian Red Cattle) and in buffalo milk, respectively. The objective of the present study was to investigate the digestion of milk from RCC using human gastrointestinal enzymes with a special focus on lipolysis, and proteolysis of the allergenic proteins, αs1-casein and β-lactoglobulin.
Mixed whole milk from nineteen RCC cows was collected from Bangladesh Livestock Research Institute dairy farm. Sampling was done from the morning milk. The animals were at different parity number and stage of lactation and the individual milk production during the sampling time varied between 1.3 to 5.0 L. The management (especially feeding) of the animals under sampling was described by Islam et al. (2014a). The samples were preserved by bronopol (1 tablet/40 mL milk; D & F control systems, Inc. Boston, MA, USA) with minimum delay after the cows were milked. All the milk samples were kept at −20°C and transferred to the Norwegian University of Life Sciences and stored at −20°C until used. Detailed composition of RCC milk including milk protein and fatty acid composition was reported by Islam et al. (2014a) and according to them the true protein and fat content of the RCC milk used herein were 38 and 42 g/kg milk, respectively.
Human GI enzymes as human gastric juices and human duodenal juices were collected and prepared according to the method of Ulleberg et al. (2011). In brief, aspiration of juices was done on six healthy, fasted (for at least 8 hrs), adult (20 to 37 years old) volunteers at Lovisenberg Diakonale Hospital, Oslo, Norway. A triple lumen tube (Maxters catheters, Marceille, France) was used for this purpose. The protocol used was approved by the Norwegian Ethical Committee.
A two phase digestion, gastric and duodenal digestion was carried out according to the method described by Devle et al. (2014) and Islam et al. (2014b). The details of the ex vivo digestion model are given in Table 1. The digestion was carried out at 37°C in a water bath for different period of time corresponding to the digestion steps (Table 1); then the reaction was stopped by placing them into −20°C (protein samples) or by adding 20 mL of chloroform and methanol (2:1) mixture and then placing into −20°C (lipid samples). The digestion experiments were performed in triplicate.
The proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using a modified method described by Islam et al. (2014b). The digested samples were mixed with the sample buffer (1:2) and applied on a precast gel (6.5 to 200 kDa; mini PROTEAN TGX precast gels, Tris Glycine extended, Bio-Rad laboratories, Inc. Made in United States) and ran for 35 min at 200 V. Then it was fixed, stained with Coomassie Brilliant Blue, destained and kept in preservation solution. Six gels were run.
A method described by Devle et al. (2014) and Islam et al. (2014b) was used to identify the protein bands in the SDS-PAGE. In brief, the identification of bands of interest was done by nano-ultra performance liquid chromatography (UPLC)/Q-Exactive Mass Spectrometry (MS), both from Thermo Fisher Scientific, Bremen, Germany. The UPLC was equipped with a trap column (Acclaim PepMap100, C18, 5 μm, 100 Å, 300 μm i.d. ×5 mm, Thermo Fisher Scientific, Bremen, Germany) and a 50 cm×75 μm analytical column (Acclaim PepMap RSLC C18, 2 μm, 100 Å, 75 μm i.d.×50 cm, nanoViper, Bremen, Germany). The Q-Exactive MS was in full scan mode (300 to 1,600 m/z) followed by (up to) 5 MS2 scans at resolution 70.000 and 35000, respectively and the used neutral collision energy was 28. For MS/MS, 1=z>5 (′z’ is the charge) precursors were excluded. An in-house Mascot (v.2.4) server was used for the database search. The data base was National Center for Biotechnology Information (NCBI), number: 20130131 (22749596 sequences; 7819872540 residues).
Peptides were identified by using the method described by Islam et al. (2014b). A nanoACQUITY UPLC (Waters, Milford, MA, USA) and quadrupole-time-of-flight (Q-TOF) Ultima MS (Micromass Ltd., Manchester, UK) was used for this purpose. The columns in the UPLC were 5-μm symmetry C18 trap column (180 μm×20 mm; Waters, USA) and 1.7-μm BEH C18 analytical column (75 μm×100 mm; Waters, USA). Identification of peptides was done in duplicate.
The multiple sequence alignment (MSA) was done to identify the minimal overlapped peptides and their position in the protein sequence. A method described by Islam et al. (2014b) was used with modifications. The software used was Clustal omega (version: CLUSTAL O (1.2.1); http://www.ebi.ac.uk/Tools/msa/clustalo/) and Jalview 2.8.0b1.
The total lipid content was first extracted and then separated by solid phase extraction (SPE) into neutral lipid (NL) and free fatty acid (FFA). The fatty acid methyl esters (FAMEs) were identified by gas chromatography-magnetic sector mass spectrometry (GC-MS). A modified method according to Devle et al. (2014) as described by Islam et al. (2014b) was followed in lipid analysis. In brief, total lipid was extracted by 20 mL of chloroform and methanol mixture (2:1) that was added immediately after the digestion. The SPE was carried-out on a liquid handling robot (Gilson, GX-274 ASPEC, Middleton, WI, USA). Elution of NL and FFA were done with 5 mL chloroform and diethyl ether:acetic acid (98:2), respectively. The FAMEs of NL and FFA were prepared by using sodium-methanolate and boron trifluride-methanol complex, respectively. In GC (Agilent 6890 series, Agilent Technology, Wilmington, DE, USA), 50 m CP-Sil 88 capillary column with ID 0.25 and 0.20 μm thickness (Varian, Middelburgh, The Netherlands) was used. The coupling Autospec Ultima MS was from Micromass Ltd. Manchester, England using electron ionization ion source (mass ranze m/z 40–600). It was done in triplicate.
The protein degradation pattern during the ex vivo digestion is shown in Figure 1. The majority of the caseins were digested after initial 20 min of gastric digestion and appeared completely digested after 40 min of gastric phase.
The whey protein serum albumin was degraded during the gastric digestion for 40 min while β-lactoglobulin and some of the α-lactalbumin were resistant. After 120 min of duodenal digestion, some β-lactoglobulin was found intact (Figure 1: lane D5, band 1) together with the fragments of serum albumin. The other bands, 2, 3 and 4 (lane D5) in Figure 1 were the blend of β-lactoglobulin, α-lactalbumin and serum albumin. In the duodenal digested samples, some bands that appear at approximately 30 to 60 kDa (a, b, c, and d in Figure 1) are the digestive enzymes present in the duodenal juices (Devle et al., 2014).
In all model digestion studies, the type, amount and activity of the enzymes, as well as pH used appear to influence the caseins digestion during the gastric phase (Almass et al., 2006). Kopf-Bolanz et al. (2012) reported a complete digestion of all the caseins after 30 min in vitro gastric digestion, while Gallier et al. (2012) showed a total degradation of caseins after 45 min using commercial enzymes of animal origin. When human gastrointestinal enzymes were used, Devle et al. (2014) reported a complete casein digestion after 40 min. The results of the present study on bovine milk are in agreement with these results. However, Islam et al. (2014; unpublished data) found traces of αs-caseins in buffalo milk after 40 min of gastric digestion. Tidona et al. (2014) reported a very low degradation of the caseins in donkey’s milk after 30 min of gastric digestion. These two findings indicate the importance of species variation and are in agreement with Inglingstad et al. (2010) in a study on human, equine, goat and bovine milk digestion.
The present study showed some intact β-lactoglobulin after 120 min of duodenal digestion and this is in line with the report on bovine milk by Devle et al. (2014), Gallier et al. (2012) and Inglingstad et al. (2010). However, Kopf-Bolanz et al. (2012) obtained almost complete digestion of bovine β-lactoglobulin by commercial gastric (120 min) and pancreatic enzymes (30, 60, 90, and 120 min). However, another important result obtained by Islam et al. (2014; unpublished data) showed almost complete hydrolysis of β-lactoglobulin in full fat buffalo milk after 5 min of duodenal digestion. So, genetic factors for the degradation of β-lactoglobulin may be of importance as was also reported by Tidona et al. (2014) in donkey milk digestion. Another factor important for the digestion of β-lactoglobulin seems to be the bile salts; ≥2 mM concentration may accelerate the digestion of the β-lactoglobulin (Gass et al., 2007). The presence of α-lactalbumin in band 2, 3, and 4 (D5, Figure 1) confirmed by UPLC-MS, indicate the hydrolysis of α-lactalbumin and Kopf-Bolanz et al. (2012) reported the complete digestion of α-lactalbumin after 30 min of duodenal digestion using commercial enzymes.
The total number of identified peptides from the different milk proteins during the different phases of ex vivo digestion is shown in Table 2. The minimal overlapped peptides from the different milk proteins after gastric and duodenal phase of digestion with their corresponding position in the protein sequence are given in Table 3 (i and ii). Maximum numbers of peptides were identified from the β-casein followed by αs1-casein, κ-casein, αs2-casein and β-lactoglobulin. Most of the peptides from κ-casein and β-lactoglobulin were identified from gastric and duodenal phase of digestion, respectively (Table 2). This is in agreement with the results obtained on buffalo milk by Islam et al. (2014; unpublished data) and Islam et al. (2014b). The sequence coverage of the identified minimal overlapped peptides (Table 3, i and ii) corresponded well with the number of total identified peptides (Table 2); β-casein showed more extensive hydrolysis, next was αs1-casein followed by κ-casein, αs2-casein and β-lactoglobulin. The presence of proline in almost all the peptides and the hydrophobicity of all the peptides are notable. These results are also in agreement with the results showed by Islam et al. (2014; unpublished data) and Islam et al. (2014b). Proline is known as a helix breaker in the protein structure and may be the proteolytic enzymes have less access to the hydrophobic sequence for further proteolysis. The extent of hydrolysis of the proteins, especially the caseins (Figure 1) was not evident neither by the number of identified peptides (Table 2) nor by the protein sequence coverage by the minimal overlapped peptides (Table 3, i and ii). This may be explained by the detection limit of the UPLC/Q-TOF MS that can identify peptides with the lowest molecular weight of 0.80 kDa. Peptides with lower molecular size as di-, tri-, and tetra-peptides and free amino acids were not detected in this study. According to Kopf-Bolanz et al. (2012) 50% of the total milk proteins were degraded into di- and tri-peptides and 10% of the proteins were degraded to the free amino acids. They also mentioned that an absence of bile salts may reduce the degradability and concluded that the size distribution of the proteins and peptides in the range of 5 kDa and tripeptides was unclear. However, the digestion conditions used in the present study and those in the study of Kopf-Bolanz et al. (2012) are different.
Milk fat consists of 95% triacylglycerol (Haug et al., 2007) and more than 95% of the milk fat can be absorbed (Mu and Hoy, 2004). But before absorption, the fat needs to be digested. The pre-duodenal (lingual and gastric) lipases and duodenal (pancreatic) lipase hydrolyze the triacylglycerol to FFAs and monoacylglycerol. These lipases attack ester bonds at sn-1 and sn-3 position of the triacylglycerol (Rogalska et al., 1990; Carriere et al., 1994; Miled et al., 2000; Armand, 2007). Different reports exist regarding the exact contribution of the pre-duodenal lipases (Carriere et al., 1993; Pafumi et al., 2002; Mu and Hoy, 2004; Gallier et al., 2013) to the total lipolysis of triacylglycerol and according to Jensen (2002) it could be 25% to 40% of the triacylglycerol.
The lipolysis of NL of RCC milk fat during the gastric and duodenal ex vivo digestion and subsequent release of FFAs are given in Figure 2. No lipolysis was observed after 40 min of gastric digestion. This is in agreement with the reported gastric lipolysis of full fat bovine milk, full fat buffalo milk, and 2% cod liver oil enriched buffalo skimmed milk (Devle et al., 2014; Islam et al., 2014; unpublished data; Islam et al., 2014b). The possible reasons are as mentioned earlier by different authors (Devle et al., 2014; Islam et al., 2014b) – firstly, the optimum pH for the gastric lipase activity is 5 to 6 (Carriere et al., 1993) and secondly, insufficient secretion of gastric lipases because the volunteers were in a semi-fasting condition and not stimulated for lipid digestion. However, it has been reported that gastric digestion of milk fat is important for further duodenal lipolysis (Jensen 2002; Ye et al., 2011; Gallier et al., 2012). A sharp rise of FFAs (33%) was shown after 30 min of duodenal digestion. The proportion of FFA after 60 min of duodenal digestion was 15.6% higher than at 30 min and the proportion after 60 and 120 min were more or less similar. The sharp increase in FFAs after 30 min duodenal digestion is in line with the results reported by Devle et al. (2014) in cows milk, Islam et al. (2014; unpublished data) in buffalo milk and Islam et al. (2014b) in 2% cod liver oil fortified buffalo skimmed milk. The bile salts concentration was 2.4 mM in the aspirates used in the present study, whereas, the aspirates used by Devle et al. (2014) had only 1.0 mM bile salts. Moreover, the milk fat globule size of the RCC milk (3.4 μm) was smaller than in the buffalo milk (12.3 μm) as reported by Islam et al. (2014a). Bile salts are important for accelarating lipolysis by creating small lipid micelles. The present study ended with 48% lipolysis of the NL after 120 min of duodenal digestion. Final lipolysis after 120 min of duodenal digestion observed in cow and buffalo milk was 40% and 35%, respectively (Devle et al., 2014; Islam et al., 2014; unpublished data). In the present study few inconsistent fatty acids (C13:0, C20:0, C17:1 n-7, C18:3 n-3, C20:4 n-6) were also observed and are included in the results presented in Figure 2 but not shown in Table 4. Part of this inconsistency may arise from the phospholipids. However, we did not take phospholipids in consideration as Devle et al. (2014) reported a non-significant digestion of the phospholipids.
The changes in the concentration of FFAs during the different steps of ex vivo digestion are given in Table 4 which also includes the lipolysis (%) of individual fatty acids. Statistical analysis revealed that the concentration of the fatty acids were significantly (p≤0.001) different in different digestion steps except C17:0. Few of them were highest in D120 step, some were found similar between D30 and D60 steps and few were found similar among D30, D60, and D120 steps. The changes in the FFA concentration stays close to the lipolysis reported in Figure 2 when the standard deviations were taken into consideration. The standard deviation of undigested, G40, D30, D60, and D120 samples were 0.10, 0.11, 5.50, 2.10, and 6.77, respectively (for Figure 2). The average lipolysis (%) of short chain fatty acids (C6:0-C8:0), medium chain fatty acids (C10:0-C16:0) and long chain fatty acids (≥C17:0) was 52.0, 33.3, and 46.5, respectively. This is in agreement with the lipase preferred positions; sn-1 and sn-3, where short chain fatty acids are more abundant followed by long chain and medium chain fatty acids (Angers et al., 1998; Blasi et al., 2008; Maansson, 2008). The unsaturated fatty acids, C14:1 n-5, C16:1 n-7, and C18:1 n-9 showed almost similar lipolysis, ranging from 31.9% to 33.7% though their presence at lipase preferred positions of the triacylglycerol is different (Blasi et al., 2008) and mainly depends on the size of the triacylglycerol (Angers et al., 1998). The lipolysis of total saturated fatty acids and total unsaturated fatty acids showed little variation, 2.3% more in total saturated fatty acids. This is in contrary to the results of cow’s milk (Devle et al., 2014), however, Islam et al. (2014; unpublished data) reported more lipolysis in total saturated fatty acids than the total unsaturated fatty acids in buffalo milk. According to Blasi et al. (2008) “saturated fatty acids were prevalently esterified in sn-3 position, while monounsaturated fatty acids in sn-2 position, with some exceptions”. The stereospecific distribution of the fatty acids in the milk fat triacylglycerol comes out with considerable variability (Parodi, 1979; Angers et al., 1998; Blasi et al., 2008; Maansson et al., 2008).
In full fat milk from Red Chittagong Cattle, all the αs-caseins were digested after 40 min of gastric digestion. However, some β-lactoglobulin was still intact after 120 min of duodenal digestion. The β-casein was degraded more extensively and contributed to the highest number of peptides. All the peptides identified from the different proteins were rich in proline along with other hydrophobic amino acids like alanine, leucine, isoleucine, valine, phenylalanie, methionine and tryptophan.
The milk fat showed 48% lipolysis. Short chain fatty acids showed higher lipolysis than the medium and long chain fatty acids and so were the total saturated fatty acids compared to the total unsaturated fatty acids. The Red Chittagong Cattle milk from Bangladesh showed a similar digestion pattern to Nordic cow’s milk (Norwegian Red Cattle).
Figure 1
Protein degradation profile of Red Chittagong Cattle milk after ex vivo gastric (G) and duodenal (D) digestion. MW, molecular weight; kDa, kilo dalton; SA, serum albumin; CN, casein; β-Lg, β-lactoglobulin; α-LA, α-lactalbumin; STD, low molecular weight marker; 0, undigested sample; G20, gastric digestion for 20 min at pH 5.0; G40, gastric digestion for 20 min at pH 2.5; D5, D30, D60 and D120, duodenal digestion for 5, 30, 60, and 120 min, respectively at pH 7.0; D5:1, β-lactoglobulin and serum albumin; D05:2, 3, and 4, β-lactoglobulin, α-lactalbumin and serum albumin; a, amylase; b, carboxypeptidase, chymotrypsin, elastase, lipase, gastricsin and amylase; c, carboxypeptidase, elastase, lipase, trypsin and amylase; d, elastase, carboxypeptidase, chymotrypsin, amylase, lipase, and trypsin.
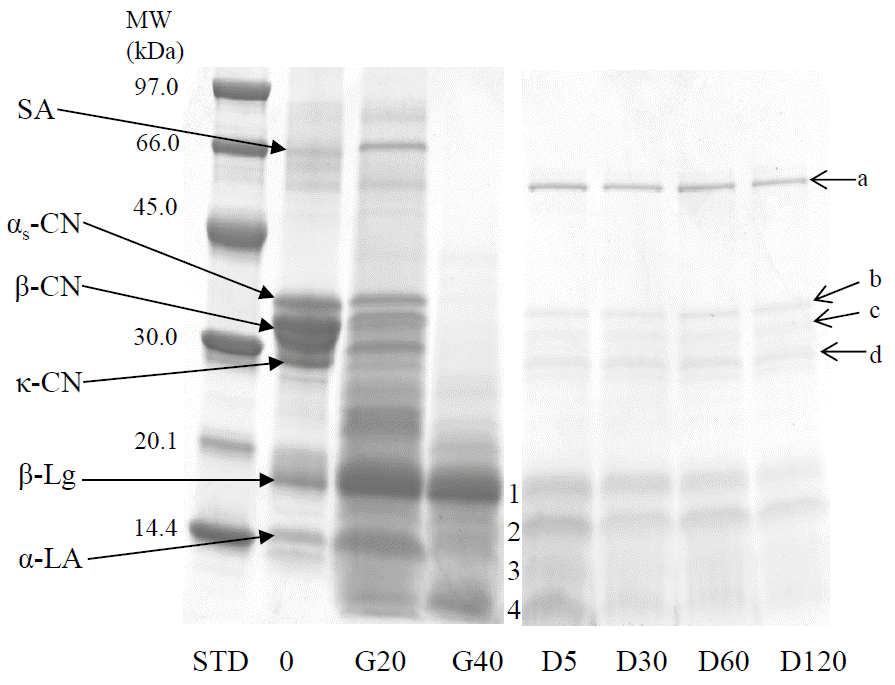
Figure 2
Proportion (%) of neutral lipid and free fatty acid during the ex vivo digestion of Red Chittagong Cattle milk. G40, gastric digestion at pH 2.5 for 20 min; D30, D60, and D120, duodenal digestion for 30, 60, and 120 min, respectively at pH 7.0. Undigested and D120 are from duplicate data where others are from triplicate data.
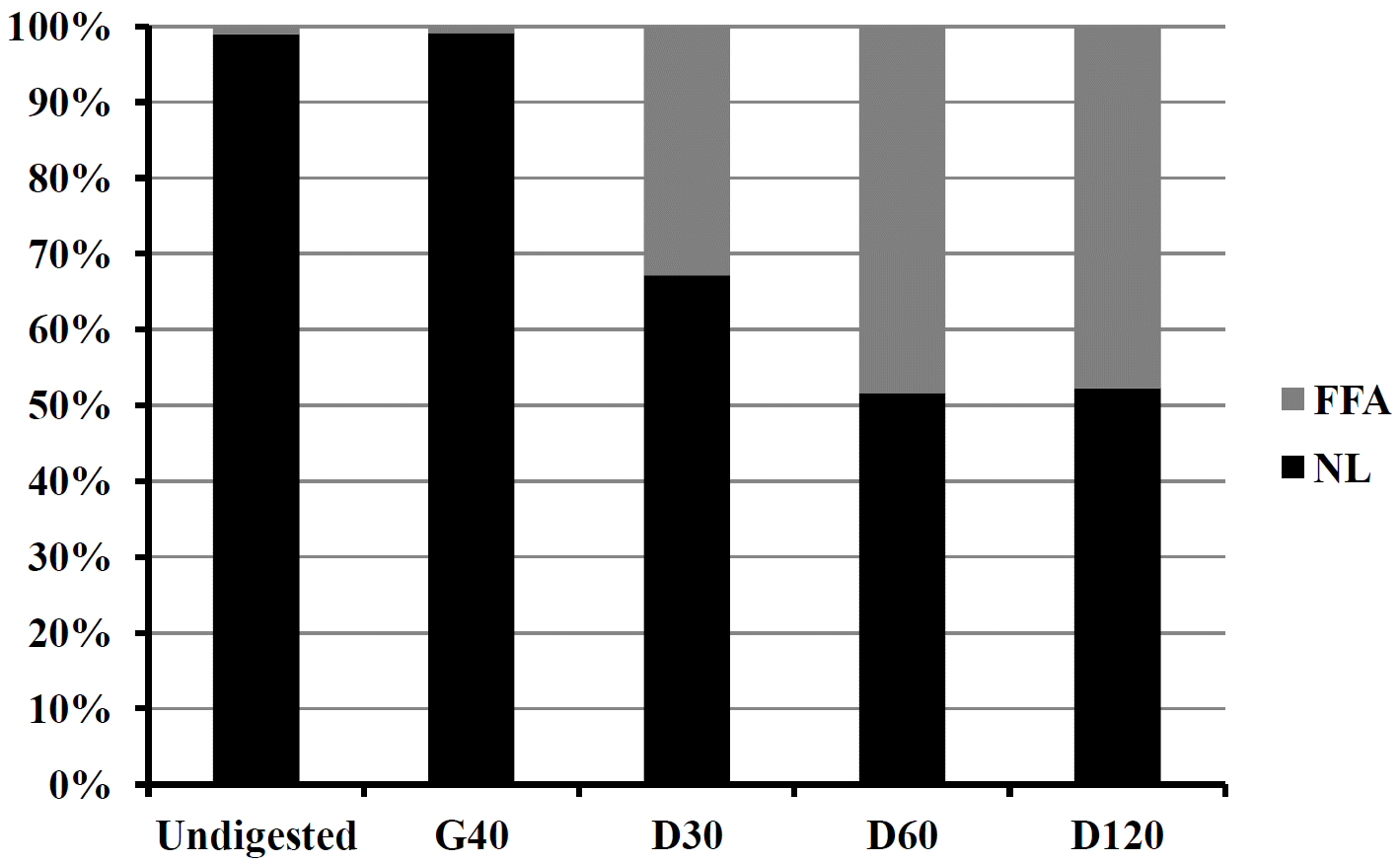
Table 1
Details on the ex vivo digestion model
Table 2
Number of peptides identified from Red Chittagong Cattle milk proteins at different stages of ex vivo digestion
| Protein | G20 | G40 | D5 | D120 |
|---|---|---|---|---|
| αs1-CN | 15 | 12 | 13 | 02 |
| αs2-CN | 04 | 04 | 07 | 01 |
| β-CN | 36 | 35 | 40 | 31 |
| κ-CN | 10 | 08 | 03 | - |
| β-Lg | 01 | 01 | 03 | 05 |
Table 3
i)
Minimal overlapped gastric and duodenal peptides from different milk proteins of ex vivo digested Red Chittagong Cattle milk
| EMW (Da) | Position1 | Peptide | Rich in ……residues2 |
|---|---|---|---|
| Gastric peptides from αs1-Casein (accession number3: B5B3R8) | |||
| 1000.452 | 165–171 | FRQFYQL | Hydrophobic and side chain containing |
| 1384.794 | 114–124 | LRLKKYKVPQL | Hydrophobic and basic |
| 1499.726 | 95–106 | HIQKEDVPSERY | Side chain containing, hydrophobic and acidic |
| 1890.858 | 141–157 | GIHAQQKEPMIGVNQEL | Hydrophobic and side chain containing |
| 1948.838 | 39–55 | FVAPFPEVFGKEKVNEL | Hydrophobic |
| 2214.998 | 195–214 | SDIPNPIGSENSEKTTMPLW | Hydrophobic and side chain containing |
| 2346.169 | 16–35 | RPKHPIKHQGLPQEVLNENL | Hydrophobic and side chain containing |
| Consensus4 | RPPPIGKEKVPQLNL | Hydrophobic, side chain containing and basic | |
| Duodenal peptides from αs1-Casein (accession number: B5B3R8) | |||
| 1236.623 | 40–50 | FVAPFPEVFGK | Hydrophobic |
| 1298.677 | 119–129 | YKVPQLEIVPN | Hydrophobic and side chain containing |
| 1336.628 | 95–105 | HIQKEDVPSER | Side chain containing, hydrophobic and acidic |
| 1956.862 | 195–213 | SDIPNPIGSENSGKTTMPL | Side chain containing and hydrophobic |
| 1965.025 | 19–35 | HPIKHQGLPQEVLNENL | Hydrophobic and side chain containing |
| 2019.977 | 140–157 | EGIHAQQKEPMIGVNQEL | Hydrophobic and side chain containing |
| Consensus | IPQPEGVPNEGVEL | Side chain containing, hydrophobic and acidic | |
| Gastric peptides from αs2-Casein (accession number: P02663) | |||
| 1196.68 | 181–189 | KISQRYQKF | Hydrophobic and side chain containing |
| 1221.59 | 58–67 | VVRNANEEEY | Hydrophobic, side chain containing and acidic |
| 2831.49 | 114–138 | LYQGPIVLNPWDQVKRNAVPITPTL | Hydrophobic and side chain containing |
| 2924.65 | 111–135 | LQYLYQGPIVLNPWDQVKRNAVPIT | Hydrophobic and side chain containing |
| Consensus | LYQGPIVLNPWDQVKRNAVPIT | Hydrophobic and side chain containing | |
| Duodenal peptides from αs2-Casein (accession number: P02663) | |||
| 1245.61 | 85–94 | KITVDDKHYQ | Side chain containing, hydrophobic and acidic |
| 1733.88 | 166–179 | TKLTEEEKNRLNFL | |
| 1738.81 | 96–109 | ALNEINQFYQKFPQ | Hydrophobic and side chain containing |
| 2039.03 | 114–130 | LYQGPIVLNPWDQVKRN | Hydrophobic and side chain containing |
| 2044.98 | 111–127 | LQYLYQGPIVLNPWDQV | Hydrophobic and side chain containing |
| Consensus | LYQGPIKLNEWDQVYQNF | Hydrophobic and side chain containing | |
| Gastric peptides from β-Casein (accession number: P02666) | |||
| 1243.66 | 109–120 | GVSKVKEAMAPK | Hydrophobic and basic |
| 1511.716 | 60–72 | LQDKIHPFAQTQS | Hydrophobic and side chain containing |
| 1624.78 | 16–29 | RELEELNVPGEIVE | Hydrophobic and acidic |
| 1873.978 | 143–158 | TDVENLHLPLPLLQSW | Hydrophobic and side chain containing |
| 2015.03 | 140–157 | LTLTDVENLHLPLPLLQS | Hydrophobic and side chain containing |
| 2178.17 | 96–115 | PVVVPPFLQPEVMGVSKVKE | Hydrophobic |
| 2253.22 | 205–224 | FLLYEQPVLGPVRGPFPIIV | Hydrophobic |
| 2876.36 | 179–204 | SLSQSKVLPVPQKAVPYPQRDMPIQA | Hydrophobic and side chain containing |
| 2881.476 | 156–180 | QSWMHQPHQPLPPTVMFPPQSVLSL | Hydrophobic and side chain containing |
| 2901.51 | 129–154 | PVEPFTESQSLTLTDVENLHLPLPLL | Hydrophobic and side chain containing |
| 3935.19 | 73–108 | LVYPFPGPIPNSLPQNIPPLTQTPVVVPPFLQPEVM | Hydrophobic and side chain containing |
| Consensus | LLYEQPVPGPIVPLPQKIPQTPVPVPPFLQPEVLGLTDVENLHLPLPLLQS | Hydrophobic and side chain containing | |
ii)
Minimal overlapped gastric and duodenal peptides from different milk proteins of ex vivo digested Red Chittagong Cattle milk (Continued)
| EMW (Da) | Position1 | Peptide | Rich in ……residues2 |
|---|---|---|---|
| Duodenal peptides from β-Casein (accession number3: P02666) | |||
| 1470.67 | 56–67 | TEDELQDKIHPF | Hydrophobic, acidic and side chain containing |
| 1624.78 | 16–29 | RELEELNVPGEIVE | Hydrophobic and acidic |
| 1887.01 | 139–155 | SLTLTDVENLHLPLPLL | Hydrophobic and side chain containing |
| 1893.94 | 206–222 | LLYQEPVLGPVRGPFPI | Hydrophobic and side chain containing |
| 1993.08 | 207–224 | LYQEPVLGPVRGPFPIIV | Hydrophobic and side chain containing |
| 2004.98 | 143–159 | TDVENLHLPLPLLQSWM | Hydrophobic and side chain containing |
| 2277.06 | 121–139 | HKEMPFPKYPVEPFTESQS | Hydrophobic and side chain containing |
| 2681.28 | 156–178 | QSWMHQPHQPLPPTVMFPPQSVL | Hydrophobic and side chain containing |
| 3935.10 | 73–108 | LVYPFPGPIPNSLPQNIPPLTQTPVVVPPFLQPEVM | Hydrophobic and side chain containing |
| Consensus4 | LLDQENLHGPVRGPFPILIEMPQPPVEPFLQEQS | Hydrophobic and side chain containing | |
| Gastric peptides from κ-Casein (accession number: P02668) | |||
| 1796.97 | 39–53 | FSDKIAKYIPIQYVL | Hydrophobic and side chain containing |
| 1267.59 | 52–60 | VLSRYPSYGLN | Side chain containing, hydrophobic |
| 1197.58 | 117–126 | ARHPHPHLSF | Hydrophobic and side chain containing |
| 1108.51 | 63–71 | YYQQKPVAL | Hydrophobic and side chain containing |
| 1536.84 | 88–100 | VRSPAQILQWQVL | Hydrophobic and side chain containing |
| 2861.30 | 72–96 | INNQFLPYPYYAKPAAVRSPAQILQ | Hydrophobic and side chain containing |
| Consensus | PYKPVAVRSPAQILQ | Hydrophobic and side chain containing | |
| Duodenal peptides from β-lactoglobulin (accession number: P02754) | |||
| 1942.94 | 57–73 | VYVEELKPTPEGDLEIL | Hydrophobic and acidic |
| 1634.71 | 141–154 | TPEVDDEALEKFDK | Acidic and hydrophobic |
| 1064.53 | 108–116 | VLVLDTDYK | Hydrophobic |
| Consensus | VKTPEDEL | Acidic and hydrophobic | |
Table 4
Individual fatty acid (FA) concentrations (mean±STD μg/mL milk) in the free fatty acid fraction of Red Chittagong Cattle milk during digestion with human gastric (G) and duodenal (D) juices, and their lipolysis
REFERENCES
Abd El-Salam MH, El-Shibiny S. 2011. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci Technol 91:663–699.

Almaas H, Cases A-L, Devold TG, Holm H, Langsrud T, Aabakken L, Aadnoey T, Vegarud GE. 2006. In vitro digestion of bovine and caprine milk by human gastric and duodenal enzymes. Int Dairy J 16:961–968.

Almaas H, Eriksen E, Sekse C, Comi I, Flengsrud R, Holm H, Jensen E, Jacobsen M, Langsrud T, Vegarud GE. 2011. Antibacterial peptides derived from caprine whey proteins, by digestion with human gastrointestinal juice. Br J Nutr 106:896–905.


Angers P, Tousignant E, Boudreau A, Arul J. 1998. Regiospecific analysis of fractions of bovine milk fat triacylglycerols with the same partition number. Lipids 33:1195–1201.


Armand M. 2007. Lipases and lipolysis in the human digestive tract: where do we stand? Curr Opin Clin Nutr Metab Care 10:156–164.


Blasi F, Montesano D, De Angelis M, Maurizi A, Ventura F, Cossignani L, Simonetti MS, Damiani P. 2008. Results of stereospecific analysis of triacylglycerol fraction from donkey, cow, ewe, goat and buffalo milk. J Food Compost Anal 21:1–7.

Carriere F, Barrowman J, Verger R, Laugier R. 1993. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology 105:876–888.


Carriere F, Gargouri Y, Moreau H, Ransac S, Rogalska E, Verger R. 1994. Gastric lipases: cellular, biochemical and kinetic aspects. Lipases: Their Structure, Biochemistry and Application. Woolley P, Petersen SB, editorsCambridge University Press; New York, USA: p. 181–205.
Devle H, Ulleberg EK, Naess-Andresen CF, Rukke E-O, Vegarud GE, Ekeberg D. 2014. Reciprocal interacting effects of proteins and lipids during ex vivo digestion of bovine milk. Int Dairy J 36:6–13.

Furlund CB, Ulleberg EK, Devold TG, Flengsrud R, Jacobsen M, Sekse C, Holm H, Vegarud GE. 2013. Identification of lactoferrin peptides generated by digestion with human gastrointestinal enzymes. J Dairy Sci 96:75–88.


Gallier S, Ye A, Singh H. 2012. Structural changes of bovine milk fat globules during in vitro digestion. J Dairy Sci 95:3579–3592.


Gallier S, Cui J, Olson TD, Rutherfurd SM, Ye A, Moughan PJ, Singh H. 2013. In vivo digestion of bovine milk fat globules: Effect of processing and interfacial structural changes. I. Gastric digestion. Food Chem 141:3273–3281.


Gass J, Vora H, Hofmann AF, Gray GM, Khosla C. 2007. Enhancement of dietary protein digestion by conjugated bile acids. Gastroenterology 133:16–23.


Haug A, Hostmark AT, Harstad OM. 2007. Bovine milk in human nutrition–a review. Lipids Health Dis 6:25



Hur SJ, Lim BO, Decker EA, McClements DJ. 2011. In vitro human digestion models for food applications. Food Chem 125:1–12.

Inglingstad RA, Devold TG, Eriksen EK, Holm H, Jacobsen M, Liland KH, Rukke EO, Vegarud GE. 2010. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci Technol 90:549–563.

Islam MA, Alam MK, Islam MN, Khan MAS, Ekeberg D, Rukke EO, Vegarud GE. 2014a. Principal milk components in buffalo, holstein cross, indigenous cattle and Red Chittagong Cattle from Bangladesh. Asian Australas J Anim Sci 27:886–897.



Islam MA, Ekeberg D, Rukke EO, Vegarud GE. 2014b. Ex vivo digestion of omega-3 enriched buffalo skimmed milk. J Func Foods In press10.1016/j.jff.2014.08.016

Jensen RG. 2002. The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci 85:295–350.


Kopf-Bolanz KA, Schwander F, Gijs M, Vergeres G, Portmann R, Egger L. 2012. Validation of an in vitro digestive system for studying macronutrient decomposition in humans. J Nutr 142:245–250.


Medhammar E, Wijesinha-Bettoni R, Stadlmayr B, Nilsson E, Charrondiere UR, Burlingame B. 2012. Composition of milk from minor dairy animals and buffalo breeds: a biodiversity perspective. J Sci Food Agric 92:445–474.


Miled N, Canaan S, Dupuis L, Roussel A, Riviere M, Carriere F, de Caro A, Cambillau C, Verger R. 2000. Digestive lipases: From three-dimensional structure to physiology. Biochimie 82:973–986.


Miranda G, Mahe M-F, Leroux C, Martin P. 2004. Proteomic tools to characterize the protein fraction of Equidae milk. Proteomics 4:2496–2509.


Pafumi Y, Lairon D, de la Porte PL, Juhel C, Storch J, Hamosh M, Armand M. 2002. Mechanisms of Inhibition of Triacylglycerol Hydrolysis by Human Gastric Lipase. J Biol Chem 277:28070–28079.


Parodi PW. 1979. Stereospecific distribution of fatty acids in bovine milk fat triglycerides. J Dairy Res 46:75–81.

Rogalska E, Ransac S, Verger R. 1990. Stereoselectivity of lipases. II. Stereoselective hydrolysis of triglycerides by gastric and pancreatic lipases. J Biol Chem 265:20271–20276.


Tidona F, Criscione A, Devold TG, Bordonaro S, Marletta D, Vegarud GE. 2014. Protein composition and micelle size of donkey milk with different protein patterns: Effects on digestibility. Int Dairy J 35:57–62.

Ulleberg EK. 2011. In vitro Digestion of Caprine Whey Proteins by Human Gastrointestinal Juices: Effect of Whey Hydrolysates and Peptides on In Vitro Cell Responses. PhD thesis. Norwegian University of Life Sciences; Aas, Norway:
- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





