 |
 |
Abstract
The 5’-adenosine monophosphate-activated protein kinase (AMPK) is a key part of a kinase-signaling cascade that acts to maintain energy homeostasis. The objective of this experiment was to investigate the possible effects of fasting and refeeding on the gene expression of hypothalamic AMPK, some appetitive regulating peptides and lipid metabolism related enzymes. Seven-day-old male broiler (Arbor Acres) chicks were allocated into three equal treatments: fed ad libitum (control); fasted for 24 h; fasted for 24 h and then refed for 24 h. Compared with the control, the hypothalamic gene expression of AMPKα2, AMPKβ1, AMPKβ2, AMPKγ1, Ste20-related adaptor protein β (STRADβ), mouse protein 25α (MO25α) and agouti-related peptide (AgRP) were increased after fasting for 24 h. No significant difference among treatments was observed in mRNA levels of AMPKα1, AMPKγ2, LKB1 and neuropeptide Y (NPY). However, the expression of MO25β, pro-opiomelanocortin (POMC), corticotropin-releasing hormone (CRH), ghrelin, fatty acid synthase (FAS), acetyl-CoA carboxylase α (ACCα), carnitine palmitoyltransferase 1 (CPT-1) and sterol regulatory element binding protein-1 (SREBP-1) were significantly decreased. The present results indicated that 24 h fasting altered gene expression of AMPK subunits, appetite regulation peptides and lipometabolism related factors in chick’s hypothalamus; the hypothalamic FAS signaling pathway might be involved in the AMPK regulated energy homeostasis and/or appetite regulation in poultry.
AMPK is the central component of a cellular signaling system that monitors cellular energy change, acting as ‘metabolic master switch’ to regulate ATP concentrations in the face of stress. AMPK can be activated by allosteric effect of AMP and/or by threonine phosphorylation of one subunit in response to AMPK kinases (Hong et al., 2003; Hawley et al., 2005; Hurley et al., 2005; Hardie, 2006). In some cases, activation of AMPK requires phosphorylation of T-172 by an upstream protein kinase such as LKB1 (Hawley et al., 1996). LKB1 is a serine/threonine protein kinase which was first discovered in the study of Peutz-Jeghers syndrome (Hemminki et al., 1998; Jenne et al., 1998). LKB1 is a complex with two accessory proteins, pseudokinase STRAD (alpha or beta isoforms) and scaffold protein MO25 (alpha and beta isoforms) (Bass et al., 2003; Boudeau et al., 2003). AMPK can be activated in a variety of physiological circumstances, including hypoglycemia (da Silva Xavier et al., 2000), ischemia (Marsin et al., 2000), heat shock (Corton et al., 1994) and exercise (Mu et al., 2001; Musi et al., 2001). AMPK is also affected by several orexigenic and anorexigenic signals in the hypothalamus (e.g. thyroid hormones, ghrelin, insulin and leptin) (Kola et al., 2006). AMPK is widely expressed in the brain, including the areas that control feed intake and neuroendocrine function. Immunostaining revealed that various AMPK isoforms distributed in hypothalamus and the hindbrain (Turnley et al., 1999). In mammals, AMPK exists as a heterotrimeric enzyme complex consisting of one catalytic (alpha) subunit and two regulatory (beta and gamma) subunits (Mitchelhill et al., 1994). There are two known alpha subunit isoforms (alpha-1 and alpha-2, Stapleton et al., 1996), two beta subunit isoforms (beta-1 and beta-2, Stapleton et al., 1997) and three gamma subunit isoforms (gamma-1, gamma-2 and gamma-3, Cheung et al., 2000).
Previous studies in mammals showed that hypothalamic AMPK integrates nutrient and hormonal signals from both anorexigenic and orexigenic pathways to regulate feed intake (Obici et al., 2003; Andersson et al., 2004; Minokoshi et al., 2004). Minokoshi et al. (2004) reported that fasting resulted in increased hypothalamic AMPK activity, whereas refeeding inhibited it (Minokoshi et al. 2004). AMPK is considered as a major regulator of fatty acid synthesis pathway due to its function to phosphorylate and inactivate key enzymes in fatty acid metabolism such as acetyl-CoA carboxylase (ACC). Pharmacological inhibition of fatty acid synthase (FAS) produces anorexia in mice (Loftus et al., 2000). It is reported that the activation of AMPK in the hypothalamus is associated with activation of mitochondrial enzyme carnitine palmitoyltransferase-1 (CPT1), leading to a decrease in the cellular levels of long-chain acyl-CoAs and increase feed intake (Andersson et al., 2004). The modest reduction of long-chain acyl-CoAs within the arcuate nucleus (ARC) would increase the expression of both agouti-related peptide (AgRP) and neuropeptide Y (NPY) and enhance feed intake (Obici et al., 2003).
There have been numerous reports showing that AMPK is involved in appetite regulation in mammals (Claret et al., 2007; Dzamko et al., 2010). Because feeding behavior and energy homeostasis are basic processes crucial to the survival of all animals (Richards and Proszkowiec-Weglarz, 2007), it is logical to assume that AMPK should be involved in poultry feed intake control. Gene expression of AMPK in chicken embryos has been explored (Proszkowiec-Weglarz and Richards, 2009), but information of its expression in chicks hypothalamus remains scarce. Previous studies in mammals showed that isoform composition of AMPK subunit changes during physiological and pathological conditions (Gregor et al., 2006; Kim et al., 2009). The aim of the present experiment was to investigate if AMPK subunits (α, β and γ) gene expression is modulated by 24 h fasting in meat-type chicks and to determine the interrelationship between AMPK and the mRNA levels of some hypothalamic feeding regulatory neuropeptides (i.e. corticotropin-releasing hormone (CRH), neuropeptide Y (NPY), agouti-related peptide (AgRP), proopiomelanocortin (POMC)) and fatty acid synthase (FAS).
Male broiler (Arbor Acres) chicks were obtained from a local hatchery at 1 d of age and reared in an environmentally controlled room. The brooding temperature was maintained at 35°C (65% RH) for the first 2 d, and then decreased gradually to 31°C (45% RH) until 10 d. The light regime was 23 L/1 D. All chicks received a starter diet with 21.5% crude protein and 12.33 MJ/kg of metabolizable energy (Zhao et al., 2009). All the birds had free access to feed and water during the rearing period. The study was approved by the University and carried out in accordance with the “Guidelines for Experimental Animal” of Ministry of Science and Technology (Beijing, P. R. China). All the chicks were cared for in accordance with the Guide to the Care and Use of Experimental Animals (Olfert et al., 1993).
Seven-day-old male broiler chicks were allocated into three equal treatments: control, fed ad libitum (C); fasted for 24 h (S24); fasted for 24 h and then refed for 24 h (S24R24). At the end of each treatment period, birds were sacrificed by exsanguination (Close, 1997), and then the whole hypothalamus was collected. After snap frozen in liquid nitrogen, the tissue samples were stored at −80°C for RNA extraction.
The expression of genes in hypothalamus was quantified using quantitative real-time PCR with SYBR Green I labeling. Total RNA was isolated using the guanidinium isothiocyanate method with Trizol Reagent (Invitrogen,San Diego, CA, USA). The quality of the RNA was tested by electrophoresis on an agarose-gel and the quantity of the RNA was determined with biophotometer (Eppendorf, Germany).
RT reactions (10 μl) consisted of 500 ng total RNA, 5 mmol/L MgCl2, 1 μl RT buffer, 1 mmol/L dNTP, 2.5 U AMV, 0.7 nmol/L oligo d(T) and 10 U Ribonuclease inhibitor (TaKaRa Biotechnology, Co., Ltd. Dalian, P. R. China). Real-time PCR analysis was conducted using the Applied Biosystems 7500 Real-time PCR System (Applied Biosystems, Foster, CA, USA). Each RT-reaction served as a template in a 20 μl PCR reaction containing 0.2 μmol/L of each primer and SYBR green master mix (Takara Biotechnology, Co., Ltd. Dalian, P. R. China). Primer-set sequences are described in Table 1. Real-time PCR reactions were performed at 95°C for 10 s, followed by 40 cycles at 95°C for 5 s and 60°C for 34 s. SYBR green fluorescence was detected at the end of each cycle to monitor the amount of PCR product. When calculating the efficiency of qPCR primers, a standard curve was made in 10 fold dilutions, and its slope was used to calculate efficiency.
The relative amount of mRNA for a gene was calculated according to the method of Livak and Schmittgen (Livak and Schmittgen, 2001). The mRNA levels of these genes were normalized to 18s rRNA levels (ΔCT) (Proszkowiec-Weglarz et al., 2006). The ΔCT was calibrated against an average of the control chicks. The linear amount of target molecules relative to the calibrator was calculated by 2−ΔΔCT. Therefore, all gene transcription results are reported as the n-fold difference relative to the calibrator. Specificity of the amplification product was verified with melt curve.
Data are presented as means±SEM. Homogeneity of variances among groups was confirmed using Bartlett’s test. All data were subjected to one-way ANOVA analysis testing the main effect of the treatment ((Version 8e, SAS Institute, Cary, NC, USA). When the main effect of treatment was significant, differences between means were assessed by Duncan’s multiple range analysis. p<0.05 was considered significant.
Fasting for 24 h increased the gene expression of AMPKα2, AMPKβ1, AMPKβ2, AMPKγ1, STRADβ and MO25α (p<0.05), but showed no significant effects on the gene expression of AMPKα1, AMPKγ2 and LKB1 (p>0.05). The gene expression of MO25β was decreased (p<0.05) by 24 h fasting. Refeeding for 24 h restored the gene expression of AMPKα2, AMPKγ1 and MO25α to the control levels (Figure 1).
Fasting for 24 h had no significant (p>0.05) effects on gene expression of NPY. However, mRNA levels of POMC, CRH and ghrelin were significantly (p<0.05) lower during fasting compared to fed control. AgRP gene expression was significantly increased (p<0.05) by 24 h fasting. After 24 h of refeeding, mRNA levels of POMC and ghrelin were significantly increased (p<0.05). Refeeding decreased mRNA levels of NPY and restored gene expression of AgRP to control levels (p<0.05, Figure 2).
Fasting for 24 h significantly (p<0.05) decreased ACC, FAS, CPT-1 and SREBP-1 mRNA levels compared to ad libitum fed controls (Figure 3). Refeeding for 24 h restored ACC, FAS, CPT-1 and SREBP-1 gene expression; in some cases (ACC, FAS, SREBP-1), increased expression levels were significantly above the control levels, which indicated that there was a classical ‘overshoot’ response (p<0.05).
AMPK has emerged as a nutrient and glucose sensor in the hypothalamus (Momcilovic et al., 2006). It is demonstrated that hypothalamic AMPK activity increases during fasting and decreases during refeeding in mammals (Culmsee, 2001; Minokoshi et al., 2004). AMPK switches on the oxidative metabolism of glucose and fatty acids to generate ATP, while switching off ATP consuming pathways. It achieves this metabolic switching both by direct phosphorylation of metabolic enzymes and via effects on transcription (Hardie and Hawley, 2001; Hardie et al., 2003). However the information of avian AMPK remains obscure, especially its definite roles in avian energy and appetite homeostasis.
In the present study, the expression of AMPK subunits increased after fasting for 24 h, except for α1 and γ2. It has been shown that AMPK subunit genes have tissue-specific expression and both alpha subunit isoforms (alpha-1 and alpha-2) were co-expressed in all tissues examined (Proszkowiec-Weglarz et al., 2006). The current results were inconsistent with some previous reports in chicken liver (Proszkowiec-Weglarz et al., 2009). Whether this difference derived from the tissue specific expression of AMPK needs further investigation. Based on the present results, AMPK α1 was less sensitive to different nutritional conditions than AMPK α2 in the chick’s hypothalamus. When chicks were refed, the increased gene expression of AMPK subunits were reduced except AMPK γ1. The integration of these data implied that the different AMPK subunits did not respond to the fasting in the same way, which revealed the complexity of the function of AMPK in the chick’s hypothalamus. However, phosphorylation reaction of AMPK protein(s) would be a key of the activation and inactivation of AMPK signaling and the relationships among changes in gene expression of AMPK subunits and its protein phosphorylation during fasting needs further investigation.
LKB1 is one of the AMPK upstream protein kinases (Hawley et al., 1996). Hawley et al. (2003) demonstrated that complexes of LKB1 tumor suppressor, STRAD and MO25 are upstream kinases in the AMPK cascade. In chicken, a functional LKB1/AMPK pathway, similar to the corresponding pathway in mammals has been suggested (Proszkowiec-Weglarz et al., 2006). The hypothalamus of chicken expresses high amount of LKB1, MO25α and both isoforms of STRAD (Proszkowiec-Weglarz et al., 2006). In the present study, the gene expression of MO25β was decreased with 24 h fasting; however, the gene expression of STRADβ and MO25α were increased. The Increased gene expression of STRADβ and MO25α might result in the up-regulation of AMPK α2 in the chick’s hypothalamus.
Multiple neuron populations distributed throughout the brain influence the decision to seek and consume food (Morton et al., 2006). It is known that hypothalamic melanocortin system (HMS) has a crucial rule in feeding regulatory neural circuitry. HMS is composed of two different populations of neurons, one set that expresses neuropeptides Y (NPY) and agouti-related protein (AgRP) and a second set that expresses proopiomelanocortin (POMC), a precursor containing α-melanocyte-stimulating hormone (Richards et al., 2010). In the present study fasting broiler chicks for 24 h did not change mRNA level of NPY in the hypothalamus of broiler chicks, however the mRNA level of AgRP and POMC were increased and decreased respectively (Figure 2).
It is demonstrated that AgRP increase food intake when injected into the brain (Ollmann et al., 1997). Moreover, Takahashi and Cone (2005) discovered that the firing rate of AgRP neurons is elevated in brain slices from food-deprived mice. Hahn et al. (1998) suggested that hypothalamic AgRP neuron constitute a unique cell type that is activated by fasting to stimulate food intake via a simultaneous decrease of melanocortin. In contrast, genetic and pharmacologic evidence suggest that POMC neurons inhibit feeding by releasing α-melanocyte-stimulating hormone, a melanocortin receptor agonist (Tsujii and Bray, 1989; Yaswen et al., 1999). It is suggested that opposite trend of AgRP and POMC gene expression implies that AgRP neurons inhibit POMC neurons in ARC (Cowley et al., 2001; Roseberry et al., 2004).
In the present study, fasting resulted in a decrease in the mRNA level of ghrelin and CRH in the hypothalamus of broiler chicks. It is known that ghrelin acts as orexigenic peptide in mammals when injected centrally and peripherally (Nakazato et al., 2001; Date et al., 2002). In the case of mammals, orexigenic activity of ghrelin is mediated by activation of other orexigenic peptides such as NPY, AgRP and orexin (Toshinai et al., 2003). In neonatal chickens, intracerebroventricular (icv) administration of ghrelin potently inhibits food intake in a dose dependent manner (Khan et al., 2006). Saito et al. (2005) suggested that anorexigenic effects of central ghrelin mediated via CRH in chickens.
All isoforms of AMPK are expressed in neuronal tissues, including these involved in the control of food intake and neuroendocrine function, such as the hypothalamus and the hindbrain (Turnley et al., 1999; Kola, 2008). Pharmacological activation of AMPK in rodent hypothalamus with 5-aminoimidazole-4-carboxamide riboside (AICAR) causes an increase in food intake (Xue and Kahn, 2006). In line with this, it has been also recently reported that AMPKβ1 KO mice reduce food intake, either under low fat (LFD) or HFD (Dzamko, 2010). Deletion of AMPKα2 in AgRP neurons led to the development of an age-dependent lean phenotype (Claret et al., 2007; Lim et al., 2010). In the present study, the enhanced gene expression of α2, β1, β2 and γ1 isoforms of AMPK was accompanied by a reduction in the mRNA levels of POMC and ghrelin and an enhancement of the AgRP mRNA levels in the hypothalamus of broiler chicks after 24 h fasting. Based on these results, it was speculated that AMPK might be involved in the appetite regulation in the hypothalamus of the chicks. AMPK might have enhanced the appetite of chicks via increasing the orexigenic gene expression of AgRP and decreasing the anorexigenic gene expression of POMC in chicks with 24 h fasting.
The level of metabolic flux through the fatty acid biosynthetic pathway in hypothalamic neurons as regulated by AMPK determines the levels of two key metabolites, malonyl-CoA, and long chain fatty acyl-CoA, which in turn lead to changes in feed intake and energy expenditure by altering the expression of orexigenic and anorexigenic neuropeptides in melanocortin system neurons (Lam et al., 2005; Lane et al., 2005; He et al., 2006). AMPK can regulate the transcription and expression of FAS and ACCα in hypothalamus of mammals and avian species (Xue and Kahn, 2006; Xu et al., 2011a; Xu et al., 2011b). The AMPK inhibits the activity of ACC, the rate-limiting enzyme involved in the production of malonyl-CoA used for fatty acyl-CoA biosynthesis, and causes a reduction in this reaction, which stimulates CPT1 and reduces the flux of substrates in the fatty acid anabolic pathway (Carling et al., 2008; Lage et al., 2008). In this study, although we anticipated an increase in the mRNA level of CPT1, however, 24 h fasting significantly decreased CPT1 gene expression in the hypothalamus of broiler chicks. The underlying mechanism needs further investigation.
In our experiment, the mRNA levels of FAS and SREBP-1 were reduced after 24 h fasting and refeeding caused an overshooting of FAS and SREBP-1 gene expression. FAS inhibitor reduces food intake in mammals and chickens (Dridi et al., 2006). Feed restriction decreased gene expression of FAS in an AMPK-dependent manner in mammals (López et al., 2008) and chickens (Dridi et al., 2006; Proszkowiec-Weglarz et al., 2006). AMPK can directly decrease FAS gene transcription via regulating the gene expression of SREBP-1 in mammals and avian (Foretz et al., 2005; Shaw et al., 2005; Proszkowiec-Weglarz et al., 2009). Bennett et al. (2008) recently reported that the lipogenic enzyme gene expression ‘overshoot’ in response to refeeding with a high carbohydrate diet in mice following a fast, which was attributable to enhanced binding of SREBP-1c to SRE sites located in lipogenic enzyme target gene promoters, as well as increased co-regulatory protein recruitment. In line with previous reports, the present findings revealed that the AMPK/SREBP-1/FAS signal pathway might be involved in the 24 h fasting induced appetite regulation in chicks.
In conclusion, 24 h fasting altered gene expression of AMPK subunits, appetite regulation peptides and lipometabolism related factors in chick’s hypothalamus; the hypothalamic FAS signal pathway might be involved in the AMPK regulated energy homeostasis and/or appetite regulation in poultry.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31172226) and the Yangtz River Scholar and Innovation Research Team Development Program (Grant no. IRT0945).
Figure 1
Effect of fasting and refeeding on mRNA levels of AMP-activated protein kinase AMPKα1 (A), AMPKα2 (A), AMPKβ1 (B), AMPKβ2 (B), AMPKγ1 (C), AMPKγ2 (C), LKB1 (D), STRADβ (D), MO25α (E), MO25β (E) in hypothalamus of broiler chicks. Values are means±SEM (n = 4). a,b Means with different letters differ significantly (p<0.05).
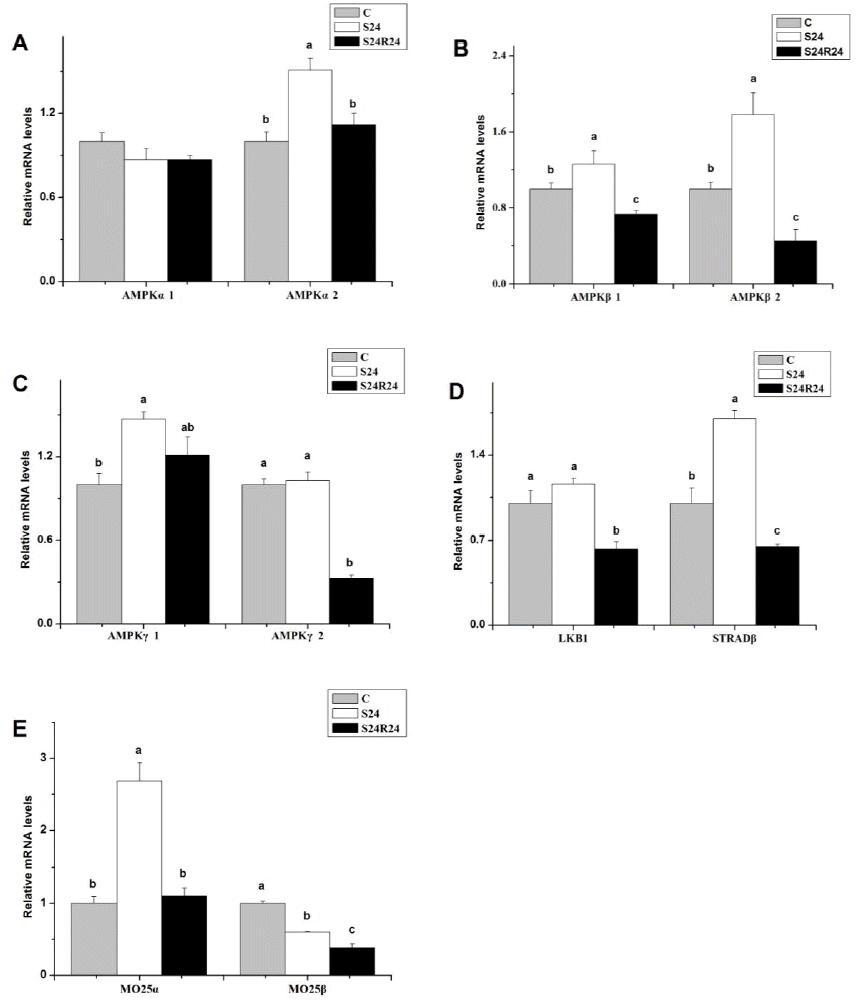
Figure 2
Effect of fasting and refeeding on mRNA levels of POMC (A), CRH (A), ghrelin (A), NPY (B) and AgRP (B) in hypothalamus of broiler chicks. Values are means±SEM (n = 4). a,b Means with different letters differ significantly (p<0.05).
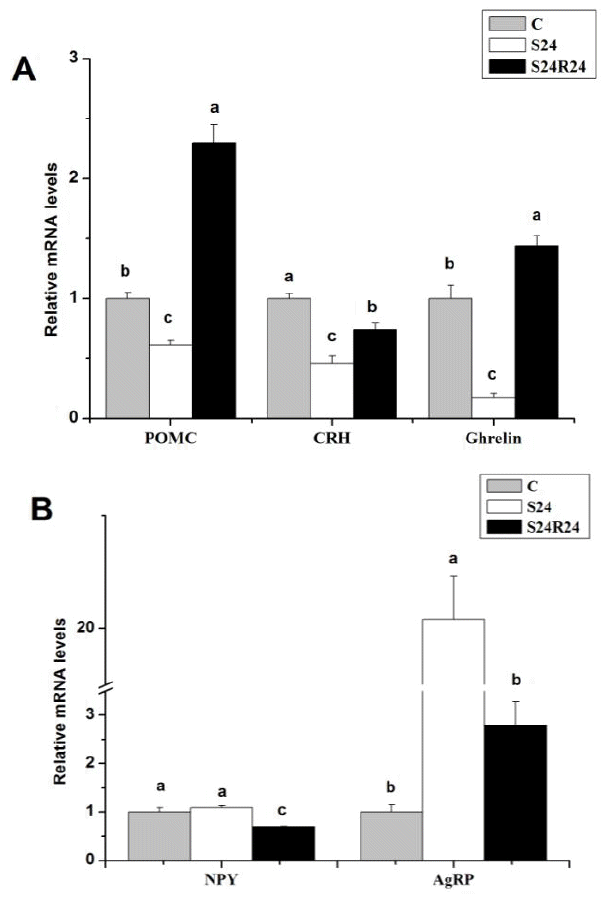
Figure 3
Effect of fasting and refeeding on mRNA levels of ACC (A), CPT-1 (A), FAS (B) and SREBP-1 (B) in hypothalamus of broiler chicks. Values are means±SEM (n = 4). a,b Means with different letters differ significantly (p<0.05).
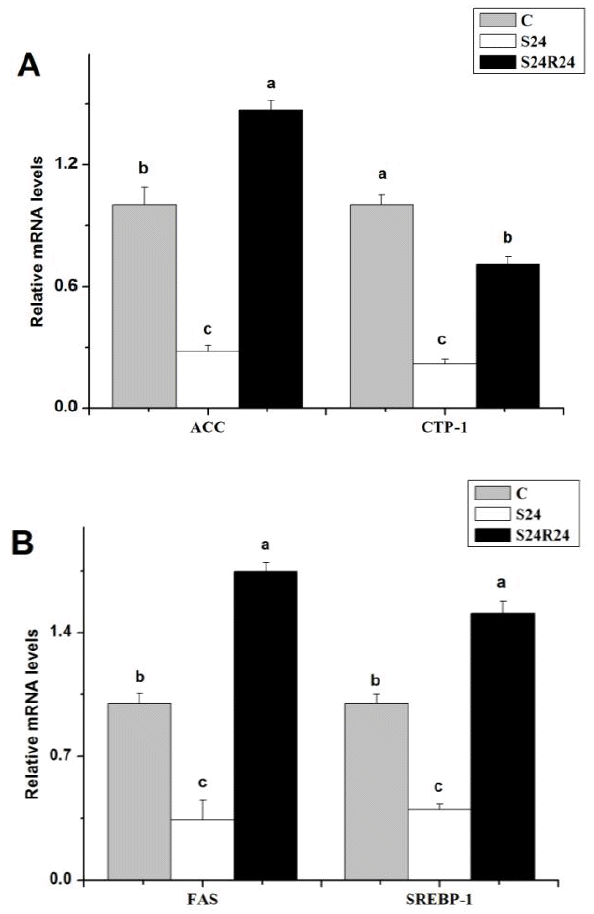
Table 1
Gene-specific primers used for the analysis of chick gene expression
18s = 18s rRNA; LKB1 = Liver kinase B1; AMPK = AMP-activated protein kinase; NPY = Neuropeptide Y; AgRP = Agouti-related peptide; CRH = Corticotropin-releasing hormone; POMC = Pro-opiomelanocortin; FAS = Fatty acid synthase; ACCα = Acetyl-CoA carboxylase α; CPT-1 = Carnitine palmitoyltransferase 1; SREBP-1 = Sterol regulatory element binding protein-1; MO25 = Mouse protein 25; STRAD = STE20 related adaptor protein; STRADβ.
REFERENCES
Bass AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, Alessi DR, Clevers HC. 2003. Activation of the tumor suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J 22:3062–3072.



Bennett M, Seo Y, Datta S, Shin D, Osborne T. 2008. Selective binding of sterol regulatory element-binding protein isoforms and co-regulatory proteins to promoters for lipid metabolic genes in liver. J Biol Chem 283:15628–15637.



Boudeau J, Bass AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. 2003. MO25 α/β interact with STRAD α/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J 22:5102–5114.



Carling D, Sanders M, Woods A. 2008. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 32:S55–S59.

Cheung P, Salt I, Davies S, Hardie D, Carling D. 2000. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 346:659–669.



Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. 2007. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117:2325–2336.



Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C. 1997. Recommendations for euthanasia of experimental animals: Part 2 DGXT of the European Commission. Lab Anim 31:1–32.


Corton JM, Gillespie JG, Hardie DG. 1994. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol 4:315–324.


Cowley M, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low ML. 2001. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484.


da Silva Xavier G, Leclerc I, Salt IP, Doiron B, Hardie DG, Kahn A, Rutter GA. 2000. Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc. Natl Acad Sci USA 97:4023–4028.



Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. 2002. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123:1120–1128.


Dridi S, Ververken C, Hillgartner FB, Lutgarde A, Van der Gucht E, Cnops L, Decuypere E, Buyse J. 2006. FAS inhibitor cerulenin reduces food intake and melanocortin receptor gene expression without modulating the other (an)orexigenic neuropeptides in chickens. Am J Physiol Regul Integr Comp Physiol 291:R138–R147.


Dzamko N, van Denderen BJ, Hevener AL, Jørgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE. 2010. AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem 285:115–122.



Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. 2005. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 54:1331–1339.


Hahn T, Breininger J, Baskin D, Schwartz M. 1998. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1:271–272.


Hardie D, Hawley S. 2001. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23:1112–1119.


Hardie DG, Scott JW, Pan DA, Hudson ER. 2003. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546:113–120.


Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. 1996. Characterization of the AMP-activated protein kinase from rat liver and identification of threonine-172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271:27879–27887.


Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. 2005. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2:9–19.


Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Höglund P, Järvinen H, Kristo P, Pelin K, Ridanpää M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. 1998. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 391:184–187.


He W, Lam T, Obici S, Rossetti L. 2006. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 9:227–233.


Hong SP, Leiper FC, Woods A, Carling D, Carlson M. 2003. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci USA 100:8839–8843.



Hurley R, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. 2005. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280:29060–29066.


Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Müller O, Back W, Zimmer M. 1998. Puetz–Jeghers syndrome is caused by mutation in a novel serine threonine kinase. Nat Genet 18:38–43.


Khan M, Dodo K, Yahata K, Nishimoto S, Ueda H, Taneike T, Kitazawa T, Hosaka Y. 2006. Intracerebroventricular administration of growth hormone releasing peptide-6 (GHRP-6) inhibits food intake, but not food retention of crop and stomach in neonatal chicks. J Poult Sci 43:35–40.

Kola B. 2008. Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol 20:942–951.



Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. 2006. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab 17:205–215.


Lane MD, Hu Z, Cha SH, Dai Y, Wolfgang M, Sidhaye A. 2005. Role of malonyl-CoA in the hypothalamic control of food intake and energy expenditure. Biochem Soc Trans 33:1063–1067.


López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodríguez-Cuenca S, Deoliveira RM, Castañeda T, Datta R, Dong JZ, Culler M, Sleeman MW, Álvarez CV, Gallego R, Lelliott CJ, Carling D, Tschöp MH, Diéguez C, Vidal-Puig A. 2008. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7:389–399.


Lage R, Diéguez C, Vidal-Puig A, López M. 2008. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med 14:539–549.


Lim CT, Kola B, Korbonits M. 2010. AMPK as a mediator of hormonal signalling. J Mol Endocrinol 44:87–97.


Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 25:402–408.


Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. 2000. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10:1247–1255.


Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574.


Mitchelhill KI, Stapleton D, Gao G, House C, Michell B, Katsis F, Witters LA, Kemp BE. 1994. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J Biol Chem 269:2361–2364.


Momcilovic M, Hong SP, Carlson M. 2006. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 281:25336–25343.


Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–295.


Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ. 2001. A role for AMP-activated protein kinase in contraction-and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094.


Musi N, Fujii N, Hirshman MF, Ekberg I, Fröberg S, Ljungqvist O, Thorell A, Goodyear LJ. 2001. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50:921–927.


Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. 2001. A role for ghrelin in the central regulation of feeding. Nature 409:194–198.


Obici S, Feng Z, Arduini A, Conti R, Rossetti L. 2003. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9:756–761.


Olfert ED, Cross BM, McWilliam AA. 1993. Guide to the care and use of experimental animals. Canadian Council on Animal Care; 1000-151 Slater Street, Ottawa, Ontario K1P 5H3:
Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. 1997. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138.


Proszkowiec-Weglarz M, Richards MP, Humphrey BD, Rosebrough RW, McMurtry JP. 2009. AMP-activated protein kinase and carbohydrate response element binding protein: a study of two potential regulatory factors in the hepatic lipogenic program of broiler chickens. Comp. Biochem. Physiol B Biochem Mol Biol 154:68–79.

Proszkowiec-Weglarz M, Richards MP, Ramachandran R, McMurtry J. 2006. Characterization of the AMP-activated protein kinase pathway in chickens. Comp Biochem Physiol B Biochem Mol Biol 143:92–106.


Proszkowiec-Weglarz M, Richards MP. 2009. Expression and activity of the 5’-adenosine monophosphate-activated protein kinase pathway in selected tissues during chicken embryonic development. Poult Sci 88:159–178.


Richards MP, Rosebrough RW, Coon CN, Mcmurtry JP. 2010. Feed intake regulation for the female broiler breeder: In theory and in practice. J Appl Poult Res 19:182–193.

Richards MP, Proszkowiec-Weglarz M. 2007. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poult Sci 86:1478–1490.


Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. 2004. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 41:711–722.


Saito E, Kaiya H, Tachibana T, Tomonaga S, Denbow D, Kangawa K, Furuse M. 2005. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chickens. Regul Pept 125:201–208.


Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. 2004. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113:274–284.



Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, DePinho RA, Montminy M, Cantley LC. 2005. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310:1642–1646.



Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, The T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. 1996. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271:611–614.


Stapleton D, Woollatt E, Mitchelhill KI, Nicholl JK, Fernandez CS, Michell BJ, Witters LA, Power DA, Sutherland GR, Kemp BE. 1997. AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Lett 409:452–456.


Takahashi KA, Cone RD. 2005. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146:1043–1047.


Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M. 2003. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 144:1506–1512.


Tsujii S, Bray GA. 1989. Acetylation alters the feeding response to MSH and beta-endorphin. Brain Res Bull 23:165–169.


Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. 1999. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72:1707–1716.


Xue B, Kahn B. 2006. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 574:73–83.



Xu P, Denbow CJ, Meiri N, Denbow DM. 2011. Fasting of 3-day-old chickens leads to changes in histone H3 methylation status. Physiol Behav 105:276–282.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





