 |
 |
Abstract
The objective of this study was to evaluate the antioxidant capacity of Lactobacillus plantarum ZLP001 and its effects on growth performance and antioxidant status in weaning piglets. The survival in hydrogen peroxide and free radical-scavenging activity of Lactobacillus plantarum ZLP001 were analysed in vitro. The Lactobacillus plantarum ZLP001 showed high viability in 1.0 mmol/L hydrogen peroxide and high scavenging ability against hydroxyl, superoxide anion, and DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals which was dose dependent. Ninety-six weaning piglets were selected (7.45±0.79 kg) and divided into three groups comprising of negative control without any supplementation, treatment group with supplemented 6.8×107 Lactobacillus plantarum ZLP001 CFU/g of diet, and positive control with antibiotic treatment (chlorotetracycline, 80 mg/kg diet). The results showed that Lactobacillus plantarum ZLP001 supplementation enhanced feed conversion rates in piglets compared with control (p<0.05). Supplementation of Lactobacillus plantarum ZLP001 increased the concentration of superoxide dismutase (p<0.05), glutathione peroxidase (p<0.01) and catalase in serum (p<0.10), while decreased the concentration of malondialdehyde (p<0.05). The present study implies that the strain Lactobacillus plantarum ZLP001 had high antioxidant ability and its supplementation improved the growth performance and antioxidant status of weaning piglets, so it can be considered useful to alleviate oxidative stress and increase productive performance of pigs. (Key Words: Probiotic, Antioxidant Capacity, Weaning Piglet)
Piglets are faced with many new stressors during the post-weaning period which can lead to risk of diarrhea, reduced growth rate, changes in gut morphology and microbiology, and increased susceptibility to disease and death (Hampson, 1994). Many studies have shown that direct-fed Lactobacillus preparation plays an important role in the improving of gut microflora balance and consequently in the better health condition of piglets. The functional effects of Lactobacillus such as protection against infections, stimulation of immune system, reduction of incidence of diarrhea, and improvement of production performance have been demonstrated in many studies (Ouwehand et al., 2002; Koninkx and Malago, 2008).
Several in vivo studies have demonstrated that some Lactobacillus strains can improve the antioxidant status of rats and humans (Kaizu et al., 1993; Kullisaar et al., 2003). Therefore, many investigations have focused on antioxidant properties of lactic acid bacteria and their role in health and disease recently (Ito et al., 2003; Kim et al., 2006). There are only few studies about the effects of Lactobacillus on the antioxidant status of pigs (Wang et al., 2009), especially on weaning piglets. However, it is known that an abnormality in the antioxidant defence system can increase the susceptibility of pigs to stress, resulting in decreased performance and reduced immune function (Duthie et al., 1989; Lauridsen et al., 1999).
The main objective of this study was to estimate the viability in hydrogen peroxide and the free radicals scavenging ability of Lactobacillus plantarum ZLP001 isolated from gastrointestinal mucosa of a healthy weaning piglet. In addition, the effects of this Lactobacilli strain on weaning piglet production performance and oxidant status were also determined.
A probiotic lactic acid bacterial strain, Lactobacillus plantarum ZLP001 was originally isolated from the gastrointestinal tract of a healthy weaning piglet in our laboratory. The strain was identified through standard morphological, biochemical and physiological tests, and by 16s rRNA gene sequence analysis by the China Center of Industrial Culture Collection (Beijing, China). Stock cultures in MRS broth were mixed with 20% of sterilized glycerol (v/v) at a concentration of 4:1 and stored at −80°C.
The Lactobacillus plantarum ZLP001 (concentration of 109 CFU/ml) was incubated with 1.0 mmol/L hydrogen peroxide at 37°C. The hydrogen peroxide concentration was determined by spectrophotometer at 230 nm absorption using the molar extinction coefficient for hydrogen peroxide. At 30-min time intervals, the aliquots were removed and plated onto MRS plates after serial ten-fold dilution and the number of viable cells was estimated. All plates were incubated at 37°C for 48 h. The effect of 1.0 mmol/L hydrogen peroxide on the survival of Lactobacillus plantarum ZLP001 was tested at 30-min time intervals within 240 min.
The scavenging activities of Lactobacillus plantarum ZLP001 was tested against the hydroxyl radical, superoxide radical, and DPPH. To this aim the Lactobacillus plantarum ZLP001 cells were harvested after 20 h of incubation. The bacterial cells were washed three times with isotonic saline and re-suspended in 0.9% NaCl. The bacterial counts in the cell pellet were adjusted to 106, 107, 108, and 109 CFU/ml.
The scavenging capacity of hydroxyl radical was estimated according to the method of de Avellar et al. (2004) modified as follows: 1 ml of 1,10-phenanthroline (0.75 mmol/L), 2 ml of phosphate buffer (pH 7.4) and 1 ml of FeSO4 (0.75 mmol/L) were thoroughly mixed. Then, 1 ml of hydrogen peroxide (0.12%) and 1 ml of the Lactobacillus plantarum ZLP001 intact cells were added. The mixture was incubated at 37°C for 90 min, and the absorbance was measured at 536 nm. The scavenging ability was measured as follows: (As−Ac)/(Ab−Ac)×100; where As represents the absorbance of the sample; Ac represents the absorbance of the control solution including 1,10-phenanthroline, FeSO4, and hydrogen peroxide; Ab represents the absorbance of the blank solution including 1,10-phenanthroline and FeSO4.
The assessment of the scavenging capacity of superoxide anion radical by Lactobacillus plantarum ZLP001 was carried out according to the methods of Zhao et al. (2003) modified as follows: the Tris-HCl buffer (1 mol/L, pH 8.2, including 0.1 mmol/L EDTA) 3 ml and Lactobacillus plantarum ZLP001 intact cells 1 ml were mixed and incubated for 25 min in 25°C water bath. Then, 40 μl of 45 mmol/L pyrogallol preheated at 25°C was added. The controls included only Tris-HCl buffer and deionized water. The absorbance of the sample and control were measured at 325 nm every 30 s for 5 min. The scavenging activity was measured as follows: (ΔAo−ΔA)×100/ΔAo; where ΔAo and ΔA are the speed of pyrogallol autoxidation before and after the addition of the sample and deionized water, respectively.
The scavenging of DPPH by Lactobacillus plantarum ZLP001 was analyzed by a modified method utilized by Shimada et al. (1992). Eight tenths of a milliliter of intact cells and 1 ml of freshly prepared DPPH solution (0.2 mmol/L in ethanol) were mixed and reacted in the dark for 30 min. Blank samples contained deionized water. The scavenged DPPH was then monitored by measuring the decrease in absorbance at 517 nm after centrifugation at 6,000 g for 10 min. The scavenging ability was defined as follows: (1−A517(sample)/A517(blank))× 100.
Ninety-six piglets (Landrance×Yorkshire, 48 males and 48 females) with 7.45±0.79 kg initial body weight (BW) were selected from Beijing Jingdongyu Farm (Beijing City, China). The pigs were weaned at 28 d of age and randomly allotted to three groups by initial BW. Four replicates per treatment were made and eight pigs per pen were allocated. Each pen was 1.65×1.45 m2 with mesh floor, a feeder and a water nipple. The pig barn was maintained at 25 to 28°C. All pigs had free access to feed and water throughout the 4-wk feeding trial. The basal diet mainly contained maize and soybean meal, and the nutrient contents met or exceeded nutrient requirements recommended by NRC (1998). The dietary treatments consisted of the basal diet with no additive served as a negative control, the basal diet with antibiotic (chlorotetracycline, 80 mg/kg diet) served as a positive control, and the basal diet with freeze dried Lactobacillus plantarum ZLP001 at 6.8×107 CFU/g of diet. Details on the ingredient composition and chemical analysis of the diets are shown in Table 1.
Piglets were weighed and the feed intake per pen was recorded every week in order to calculate the average daily weight gain, average daily feed intake, and feed conversion ratio. At the end of the experiment, blood was collected from four pigs per pen (pig closer to the average weight of the pen) by anterior vena cava puncture using 10-ml anticoagulant-free vacutainer tubes. The serum was separated by centrifugation at 3,000 g for 10 min, and was stored at −20°C until specific antioxidant indexes analysis.
Total superoxide dismutase, glutathione peroxidase, malondialdehyde, and catalase were determined using assay kits supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacture’s instructions. The superoxide dismutase activity was measured by the xanthine oxidase method. The glutathione peroxidase activity was determined by measuring the reduction of glutathione. The content of malondialdehyde was determined with 2-thiobarbituric acid. The catalase activity was determined by measuring the intensity of a yellow complex formed by molybdate and hydrogen peroxide at 405 nm after ammonium molybdate was added to terminate hydrogen peroxide degradation, which is catalysed by catalase.
Statistical analysis of the obtained data was carried out using the PROC General Linear Model procedure of SAS (1997). In the in vitro assessment, the results were expressed as the mean and standard deviation of three determinations. In the in vivo experiment, the pen was considered the experimental unit. Differences among means were tested using Tukey’s test and probability of p<0.05 was used to denote significance.
As showed in Figure 1, the survival of Lactobacillus plantarum ZLP001 decreased along with the incubation time, however Lactobacillus plantarum ZLP001 still maintained over 95% of the original viable cell numbers after 240 min of incubation (8.46 vs 8.11 log CFU/ml at 0 and 240 min, respectively).
The scavenging activities of Lactobacillus plantarum ZLP001 against the hydroxyl radical, superoxide radical, and DPPH are shown in Figure 2. It can be seen that the free radical scavenging activity of Lactobacillus plantarum ZLP001 against all three radicals increased in dose-dependent manner. In particular, Lactobacillus plantarum ZLP001 exhibited obvious inhibitory effects especially on the generation of the hydroxyl radical (83.6% of scavenging activity) and superoxide radical (78.5% of scavenging activity) with the 109 CFU/ml concentration. For the DPPH radical, Lactobacillus plantarum ZLP001 showed lower scavenging activity than the other two radicals. Only 51.1% scavenging activity was exhibited at 109 CFU/ml and almost no scavenging activity at 106 CFU/ml.
The effects of dietary Lactobacillus plantarum ZLP001 supplementation on growth performance of weaning piglets are shown in Table 2. Over the 4 wks feeding trial, piglets of antibiotic and probiotics groups were consumed higher feeds than no additive group (p<0.05). The piglets received diets containing Lactobacillus plantarum ZLP001 supplements had the same daily weight gain compared with the antibiotic group (p>0.05) and significant higher than no additive group (p<0.05). The feed conversion ratio in probiotic and antibiotic group were significantly lower (p<0.05) than those of no additive group.
The effects of Lactobacillus plantarum ZLP001 supplementation on antioxidant enzyme activity and malondialdehyde levels in serum of piglets are shown in Table 2. The activity of superoxide dismutase and glutathione peroxidase in Lactobacillus plantarum ZLP001 supplementation was significantly higher (p<0.05 and p<0.01, respectively) compared with antibiotic and no additive treatments. The malondialdehyde concentration was significantly decreased (p<0.05) for pigs fed Lactobacillus plantarum ZLP001 compared with pigs fed no additive feed. There were no significant differences (p>0.05) among the treatments regarding the activities of catalase in serum of piglets.
Lactobacillus plays significant role in the natural microflora of the pig intestinal tract. They create a healthy balance between beneficial and potentially harmful microorganisms in the gut ecosystem when they are present in sufficient numbers. The use of these cultures in pig diets is desirable for their potential probiotic effects.
The Lactobacillus plantarum ZLP001 had high viability in the presence of 1.0 mmol/L hydrogen peroxide. In the present study, the cells were viable exceed 95% after 240 min of incubation. It was not unexpected that Lactobacillus cells have high viability in hydrogen peroxide, because hydrogen peroxide is one of the antimicrobial compounds produced by Lactobacilli when it plays a role in the gastrointestinal tract (Ouwehand and Vesterlund, 1998). Our result was similar with the findings of Wang et al. (2009) who found that over 90% of Lact. fermentum cells were viable after incubation in an environment of 1.0 mmol/L hydrogen peroxide. However, other researchers also found that Lactobacilli have high viability only 180 min in the presence of 1.0 mmol/L hydrogen peroxide (Kullisaar et al., 2002). The reason for this discrepancy may be due to differences of Lactobacillus strains used or the initial number of Lactobacillus.
According to free radical chain reaction proposed by Farmer et al. (1942), free radicals formed in the process of incomplete reduction of oxygen can attack and damage biological molecules. The consequences of a free radical chain reaction can result in serious damage to living organisms. The production of free radicals and many diseases are closely related (Halliwell and Gutteridge, 1984; Halliwell and Gutteridge, 1989). Therefore, the ability of Lactobacillus plantarum ZLP001 to scavenge hydroxyl, superoxide, and DPPH radicals were determined in this study. The Lactobacillus plantarum ZLP001 showed significant ability to scavenge the hydroxyl, superoxide, and DPPH radicals and supported previous findings on scavenging ability of Lactobacillus to free radicals in vitro (Lin and Chang, 2000; Kullisaar et al., 2002). The effect of scavenging on these three radicals were all showed to be dose dependent, in fact with the concentration of 109 CFU/ml consistently exhibiting the highest and with the concentration of 106 CFU/ml consistently exhibiting the lowest scavenging ability. This tendency was consistent with studies by Wang et al. (2009) using Lact. Fermentum.
The in vitro studies for evaluating Lactobacillus antioxidant properties are far from the requirements in practice. The strain for the in vivo trial was selected primarily for the survival in environment of hydrogen peroxide and free radical scavenging activities. In this study, the selected Lactobacillus plantarum ZLP001 showed high viability in hydrogen peroxide and high free radical scavenging ability. Therefore, the in vivo trial was carried out to estimate the effects of Lactobacillus plantarum ZLP001 supplementation in weaning piglets. It was demonstrated that weaning in piglets is related to oxidative stress (Sauerwein et al., 2007). This stress can result in reduced performance and increased susceptibility to disease and death (Hampson, 1994; Cromwell, 2002). In this study, Lactobacillus plantarum ZLP001 supplementation group showed significant higher productivity effect compared with the control group. These results indicated that in terms of feed consumption, the probiotic groups consumed 2.3% and 6.1% less than the antibiotic group and no additive group to achieve the same weight respectively. Similar observations were obtained by Francisco et al. (1995) and Chang et al. (2001) that selected probiotic strains had increasing effect on feed conversion rate in piglets. This may be partly owing to the antioxidative effect of Lactobacilli strains that alleviating the oxidative stress of weaning piglets.
The antioxidant systems in the body including numerous antioxidant enzymes (i.e. superoxide dismutase, glutathione peroxidase, and catalase) that are employed to protect the body from oxidative stress (Zhan et al., 2006). The superoxide dismutase enzyme is used to ending the cascade of electron stealing which were caused by the superoxide radicals, and then alleviate the damage to the cell. Superoxide radicals are converted by superoxide dismutase to less toxic hydrogen peroxide which is further processed to form non-toxic water by either glutathione peroxidase or catalase. In this study, the antioxidant status of weaning piglet was evaluated through measuring these enzymes in serum. At the end of the experiment phase, the superoxide dismutase and glutathione peroxidase in the serum of pigs fed Lactobacillus plantarum ZLP001 was significantly higher (p<0.01) than for pigs fed the antibiotic diet and basal diet, which consisted with the studies by Wang et al. (2009) on growing-finishing pigs using Lact. fermentum. There was no significant difference (p>0.05) among three treatments on catalase concentration in the serum in this study. The serum catalase levels were 17.4% higher for piglets fed Lactobacillus plantarum ZLP001 than for piglets fed the negative control. The high activities of these enzymes will decrease the concentrations of oxygen radicals in body, thereby alleviating the oxidative damage to living organisms. Furthermore, malondialdehyde is also one of the most frequently used indicators of oxidative stress in an organism (Janero, 1990; Nielsen et al., 1997). Cellular biomembranes are one of the major targets of reactive oxygen species, where they induce lipid peroxidation resulting in the production of malondialdehyde, while malondialdehyde can react with biomolecules and exert cytotoxic and genotoxic effects (Ardestani and Yazdanparast, 2007). In this study, it was observed that malondialdehyde levels in serum of weaning piglets were significantly reduced (p<0.05) for piglets fed Lactobacillus plantarum ZLP001 compared with the basal diet. These results demonstrated that there was improvement in the antioxidant defence system for weaning piglets fed Lactobacillus plantarum ZLP001.
The results of our study suggest the antioxidative potential of Lactobacillus plantarum ZLP001. The strain used in the present study exhibited significant in vitro free radical-scavenging capacity, which was dose dependent. Supplementation in the diet improved the growth performance and antioxidant status of pigs as exhibited by improved feed conversion ratio and increased antioxidant enzymes, and decreased malondialdehyde concentration in the serum. This study suggests that Lactobacillus plantarum ZLP001 is a highly antioxidative bacterial strain, which could be used in the future to alleviate oxidative stress and thereby increase growth performance. However, the exact mechanism through which Lactobacillus plantarum may play an important role in scavenging of free radical remains uncertain and further research is also needed to fully determine the exact antioxidative mechanism of this strain through which the antioxidative effects are achieved.
Figure 1
Survival of Lactobacillus plantarum ZLP001 in the presence of hydrogen peroxide. Error bars indicate the standard deviation of three determinations.
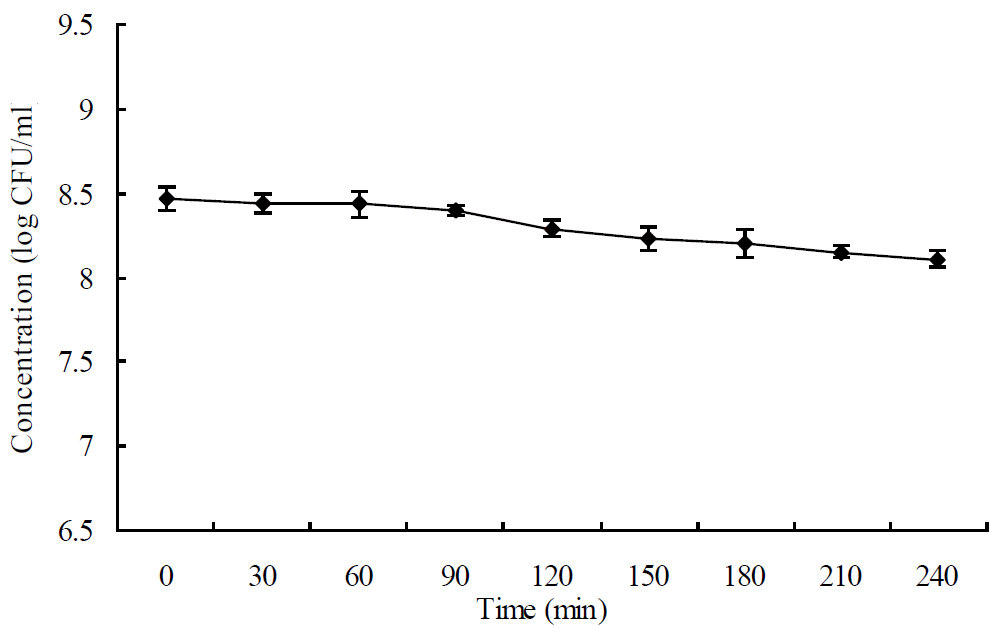
Figure 2
Scavenging activities of Lactobacillus plantarum ZLP001 on hydroxyl radical, superoxide radical, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. Error bars indicate the standard deviation of three determinations.
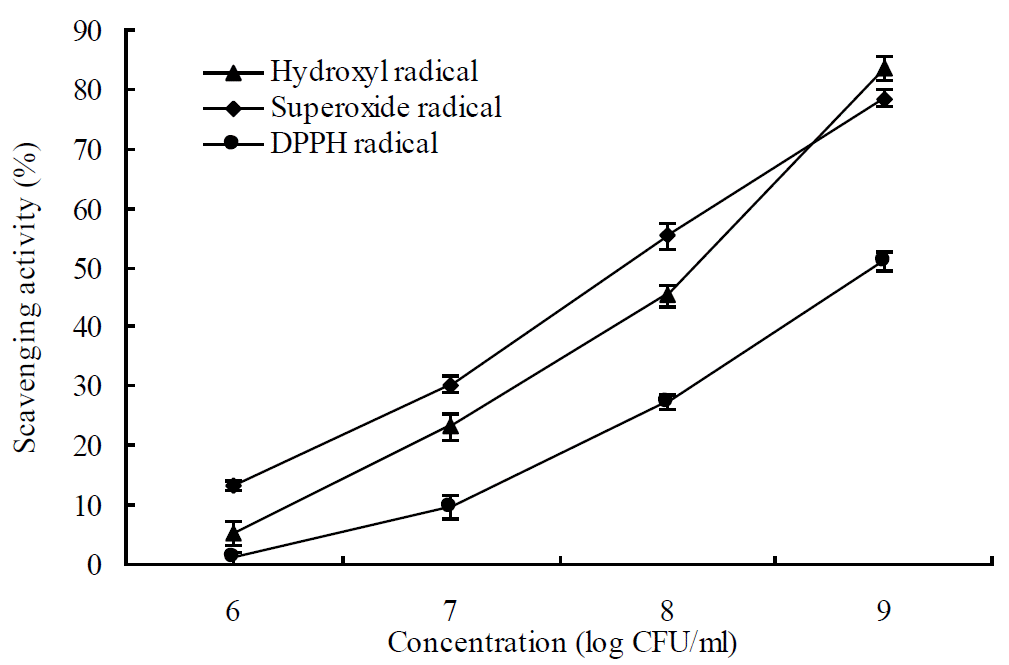
Table 1
Ingredient and composition of the basal diet
| Ingredients (g/kg) | |
|---|---|
| Corn | 600 |
| Soybean meal | 150 |
| Extruded soybean | 60 |
| Fish meal | 50 |
| Wheat bran | 50 |
| Whey | 50 |
| Premix* | 40 |
| Chemical compositions | |
| Digestible energy1 (MJ/kg) | 13.76 |
| Crude protein2 (g/kg) | 182.9 |
| Lysine2 (g/kg) | 11.8 |
| Methionine2 (g/kg) | 4.2 |
| Calcium2 (g/kg) | 9.8 |
| Total phosphorus2 (g/kg) | 7.4 |
Table 2
Effect of Lactobacillus plantarum ZLP001 supplementation on growth performance of weaning piglets and superoxide dismutase, glutathione peroxidase, malondialdehyde, and catalase concentrations in the serum
| Parameters |
Dietary treatment
|
SEM* | p value | ||
|---|---|---|---|---|---|
| No additive | Antibiotic | Lactobacillus plantarum ZLP001 (6.8×107 CFU/g of diet) | |||
| Growth performance | |||||
| Average daily feed intake (g/d) | 612b | 651a | 660a | 13.1 | 0.035 |
| Average daily gain (g/d) | 381b | 419a | 436a | 10.2 | 0.022 |
| Feed conversion ratio | 1.61a | 1.55b | 1.51b | 0.019 | 0.017 |
| Antioxidant status | |||||
| Superoxide dismutase (U/ml) | 112.9b | 122.2b | 139.8a | 5.52 | 0.043 |
| Glutathione peroxidase (U/ml) | 401.5c | 432.1b | 489.2a | 7.15 | 0.007 |
| Malondialdehyde (nmol/ml) | 6.23a | 5.49ab | 4.10b | 0.482 | 0.025 |
| Catalase (U/ml) | 5.16 | 5.71 | 6.06 | 0.386 | 0.092 |
REFERENCES
Ardestani A, Yazdanparast R. 2007. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem 104:21–29.

Chang YH, Kim JK, Kim HJ, Kim WY, Kim YB, Park YH. 2001. Selection of a potential probiotics Lactobacillus strain and subsequent in vivo studies. Antonie Van Leeuwenhoek 80:193–199.


de Avellar IG, Magalhaes MM, Silva AB, Souza LL, Leitao AC, Hermes-Lima M. 2004. Reevaluating the role of 1,10-phenanthroline in oxidative reactions involving ferrous ions and DNA damage. Biochim Biophys Acta 1675:46–53.


Duthie GG, Arthur JR, Nicol F, Walker M. 1989. Increased indices of lipid peroxidation in stress-susceptible pigs and effects of vitamin E. Res Vet Sci 46:226–230.


Farmer EH, Bloomfield GF, Sundralingam A, Sutton DA. 1942. The course and mechanism of autoxidation reactions in olefinic and polyolefinic substances, including rubber. Trans Faraday Soc 38:348–356.

Francisco T, Juan R, Erenia F, Maria LR. 1995. Response of piglets to oral administration of lactic acid bacteria. J Food Prot 58:1369–1374.


Halliwell B, Gutteridge JMC. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–4.



Halliwell B, Gutteridge JMC. 1989. Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J 1:441–445.


Hampson DJ. 1994. Postweaning Escherichia coli diarrhea in pigs. Escherichia coli in Domestic Animals and Humans. Gyles CL, editorp. 171–791. CAB International; London, England:
Ito M, Ohishi K, Yoshida Y, Yokoi W, Sawada H. 2003. Antioxidative effects of lactic acid bacteria on the colonic mucosa of iron-overloaded mice. J Agric Food Chem 51:4456–4460.


Janero DR. 1990. Malondialdehyde and thiobarbituric acid reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9:515–540.


Kaizu H, Sasaki M, Nakajima H, Suzuki Y. 1993. Effect of antioxidative lactic acid bacteria on rats fed a diet deficient in vitamin E. J Dairy Sci 76:2493–2499.


Kim HS, Chae HS, Jeong SG, Ham JS, Im SK, Ahn CN, Lee JM. 2006. In vitro antioxidative properties of Lactobacilli. Asian-Aust J Anim Sci 19:262–265.

Koninkx JFJG, Malago JJ. 2008. The protective potency of probiotic bacteria and their microbial products against enteric infections. Folia Microbiol 53:189–194.

Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. 2003. Antioxidative probiotic fermented goats’ milk decreases oxidative stressmediated atherogenicity in human subjects. Br J Nutr 90:449–456.


Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, Kilk A. 2002. Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72:215–224.


Lauridsen C, Hojsgaard S, Sorensen MT. 1999. Influence of dietary rapeseed oil, vitamin E, and copper on the performance and the antioxidative and oxidative status of pigs. J Anim Sci 77:906–916.


Lin MY, Chang FJ. 2000. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci 45:1617–1622.


Nielsen F, Mikkelsen BB, Nielsen JB, Anderson HR, Grandjean P. 1997. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem 43:1209–1214.


NRC. 1998. Nutrient Requirements of Swine. 10th EditionNational Academic Press; Washington, DC, USA:
Ouwehand AC, Salminen S, Isolauri E. 2002. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 82:279–289.


Ouwehand AC, Vesterlund S. 1998. Antimicrobial components from lactic acid bacteria. Lactic Acid Bacteria. Salminen S, Wright A, Ouwehand A, editorsp. 375–388. Marcel Dekker Inc; New York, USA:

SAS. 1997. SAS/STAT user’s guide. Version 9SAS Institute Inc; Cary, NC, USA:
Sauerwein H, Schmitz S, Hiss S. 2007. Effects of a dietary application of a yeast cell wall extract on innate and acquired immunity, on oxidative status and growth performance in weanling piglets and on the ileal epithelium in fattened pigs. J Anim Physiol Anim Nutr 91:369–380.

Shimada , Fujikawa KK, Yahara K, Nakamura T. 1992. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948.

Wang AN, Yi XW, Yu HF, Dong B, Qiao SY. 2009. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on gorwing-finishing pigs. J Appl Microbiol 107:1140–1148.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





