 |
 |
Abstract
Intrauterine growth retardation (IUGR) leads to the dysfunction in digestive system, as well as the alteration in the expression of some functional proteins. Heat shock protein 70 (Hsp70) could be induced by various stress factors, but whether Hsp70 expression is changed in neonatal IUGR infants has not been demonstrated. This study was conducted to explore the expression of Hsp70 in the liver by using the IUGR piglet model. Liver and plasma samples were obtained from IUGR and normal birth weight (NBW) piglets at birth. The neonatal IUGR piglets had significantly lower liver weight than their counterparts. The activities of aspartate aminotransferase and alanine aminotransferase in serum were enhanced significantly in IUGR indicating liver dysfunction. The activities of superoxide dismutase (p<0.01), glutathione peroxidase (p<0.01) and catalase (p>0.05) were lower and the level of malondialdehybe was higher (p<0.05) in IUGR liver compared with in NBW. According to the results of histological tests, fatty hepatic infiltrates and cytoplasmic vacuolization were present in the liver of IUGR piglets, but not in NBW liver. The expression of Hsp70 protein was significantly higher (p<0.05) in IUGR piglet liver than in NBW. Similar to where the hepatic injuries were observed, location of Hsp70 was mostly in the midzonal hepatic lobule indicating that oxidative stress might be responsible for the increased expression of Hsp70.
Intrauterine growth retardation (IUGR), usually resulting from uteroplacental vascular insufficiency, strongly increases the risk of prenatal morbidity and mortality in both human and animal infants (Aucott et al., 2004). Compared with normal birth weight (NBW) offspring, IUGR offspring have smaller organs and suffer from dysfunction in digestive system (Simmons et al., 2005; Wang et al., 2005; Lipsett et al., 2006). Additionally, the expression of some functional proteins is also altered in organs of IUGR offspring, especially in liver. For example, hepatic expression of 11β-hydroxysteroid dehydrogenase type 1 isoform, peroxisome proliferator-activated receptor gamma (PPAR-γ) coactivator 1 alpha, and phosphoenolpyruvate carboxykinase 2 mRNA were increased, while IGF-1 mRNA was decreased in fetal sheep with IUGR reduced liver growth (Gentili et al., 2009). Increased expression of PPAR-γ coactivator-1 was observed in the liver of juvenile IUGR rat and this might be associated with the programming of insulin resistance in later life (Lane et al., 2002). Lately, analysis of proteomics showed nine differentially expressed proteins in the liver of IUGR piglets, suggesting negative effect of IUGR on substrate utilization and hepatic function (Wang et al., 2008). However the expression of heat shock protein 70 (Hsp70) in the liver of IUGR offspring has not been studied.
Hsp70 plays a crucial role as intracellular cytoprotectant and molecular chaperone (Otaka et al., 2006). Hsp70 is essential for living cells to enhance tolerance to adapt environmental changes or pathogenic conditions in different organs, based on its ability to help fold newly synthesized polypeptides and degenerate damaged proteins under both physiological and stressful conditions (Robert, 2003; Otaka et al., 2006). As the prime target of tissue injury in physiological challenges (Zhang et al., 2003; Zhang et al., 2006), the liver has been demonstrated to be very sensitive to Hsp70 accumulation (Flanagan et al., 1995). Various stressors, including heat stress, ischemia-reperfusion, exposure to heavy metals and organic pollutants are able to increase the expression of Hsp70 in liver (Bedirli et al., 2004; Kanemura et al., 2009; Padmini et al., 2009). Since the redox state in IUGR offspring altered (Simmons et al., 2005; Simmons, 2006; Zadro na et al., 2009), which might induce oxidant stress, it is interesting to investigate the expression of Hsp70 in the tissues of IUGR offspring. But only a few studies have linked the expression of Hsp70 with IUGR (Wataba et al., 2004; Liu et al., 2008; Zhong et al., 2010). Considering the impact of IUGR on the whole digestive system (Trahair et al., 1997; Wang et al., 2005) and the severely impaired liver growth by IUGR during fetal period (Latini et al., 2004), our hypothesis is that the Hsp70 expression in the liver of neonatal piglets is upregulated by IUGR. Meanwhile, we also focus on the relationship between Hsp70 induction and hepatic redox state associated with IUGR.
Landrace×Large White pigs were used in this study. During the entire period of pregnancy, pregnant sows were fed daily with a 2 kg gestating diet which met the requirement (NRC, 1998), and water was offered ad libitum. At birth, 10 piglets selected from five litters were distributed into one NBW group and one IUGR group (five pigs per group), based on their birth weight. As previous described, a piglet was defined as IUGR when its birth weight was two standard deviations (SD) below the mean birth weight of the total population, while that with birth weight within one SD of the total population was defined as NBW (Wang et al., 2005; Zhong et al., 2010). Considering animal welfare and practicality, all the neonatal pigs were administered general anesthesia (50 mg/kg body weight of pentobarbital sodium) and killed by jugular exsanguinations within 2 to 4 h after birth without suckling. All experimental procedures used in the study were consistent with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee, Nanjing Agriculture University, China.
The body weights of piglets in both groups were recorded at the time of birth. During exsanguinations, blood samples were collected in tubes containing heparin anticoagulant (50 IU/ml) and then centrifuged at 1,200×g for 5 min at 4°C. The serum obtained was snap-frozen in liquid nitrogen and stored at −80°C for further analysis. The liver was isolated and removed from the piglet immediately after death. The tissue specimens for histological examination and immunohistochemistry were collected from the left lateral lobe of the liver and fixed in 4% paraformaldehyde in 100 mM phosphate buffer, pH 7.4 for 24 h, and liver samples for quantifying the levels of Hsp70 and for measuring the activities of enzymes were placed into 3 ml tubes and stored in liquid nitrogen.
The activities of total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GPx) and the level of malondialdehyde (MDA) in liver, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in serum were determined by corresponding commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The liver tissue protein concentrations were measured using the Coomassie Brilliant Blue G-250 reagent with bovine serum albumin as a standard.
The fixed liver slices were dehydrated in graded series of ethanol, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (HE). In all cases, three sections were prepared on each slide, and multiple slides were prepared per animal. Histopathologic examinations of the liver sections were conducted by three independent pathologists using light microscopy.
Paraffin-embedded liver sections (4 μm) were collected on APES-coated slides and dried at 60°C for more than 8 h. After deparaffinization and rehydration, the antigen retrieval was performed by heating the sections with 0.01 M sodium citrate buffer, pH 6.0, 10 min in a microwave oven, and then cooled at room temperature (RT) and washed in tap water. Endogenous peroxidase activity was quenched with 3% (v/v) hydrogen peroxide for 60 min at RT. Unspecific antibody binding was blocked by incubating with 1% bovine serum albumin-phosphate buffered saline (PBS, pH 7.4) for 60 min at RT. The sections were incubated in a humidified chamber with the Hsp70 primary antibody (SPA-810, Stressgen, Canada) diluted 1:100 in PBS (pH 7.4) overnight at 4°C. The Polink-2 plus™ polymer HRP detection system (Golden Bridge International Inc., USA) was used to detect the Hsp70 conjugate activity; and the sections were washed in PBS (pH 7.4) three times for 15 min. Positive cell were stain in brown color using 3, 3’-diaminobenzidine tetrachloride (Zymed Laboratories Inc., USA) as the enzyme substrate. After rinsed in tap water, the sections were counterstained with Mayer’s hematoxylin for 5 s. Slides were dehydrated, mounted and observed by conventional light microscopy. Control experiments were performed by using 1% bovine serum albumin-PBS (pH 7.4) instead of primary antibody (Li et al., 2007; Padmini et al., 2009; Zhong et al., 2010).
Approximately 0.3 g of frozen liver was homogenized in cold solution for ELISA as previously described (Zhong et al., 2010). The homogenate was centrifuged at 1,200×g for 15 min at 4°C. The supernatant fluid was collected and stored at −20°C for further analysis.
ELISA analysis was performed using commercial testing kits (goat anti-porcine Hsp70, Adlitteram Diagnostic Laboratories, USA) including the immunoreagents (e.g. antibodies, conjugates) and the 96-microwell plates with specific antibody-coated wells. Standards and specimens were dispensed into appropriate wells. An equal amount of biotin conjugate reagent was added to the wells of specimens, while the enzyme conjugate reagent was added to each well. After gently mixed using microtiter plate shaker for 15 s, the mixture was incubated at 36±2°C for 60 min. The content of each well was decanted to a waste container and the microtiter wells were rinsed five times with distilled water and tapped dry to remove the solution containing any unbound reagents. And then, 3, 3’ 5, 5’-tetramethylbenzidine substrate solution was added to each well for color development. After mixed for 15 s as before, the solution was incubated at 36±2°C for 15 min, keeping out of direct rays of the sun. The chromogenic reaction was stopped after 30 min at RT by adding stop solution and gently mixed for 30 s.
The average absorbance values for each set of reference standards, control and samples were determined at 450 nm and a standard curve was constructed. The results were expressed in nanogram per milliliter according to the calibration curve obtained from serial dilutions of Hsp70 and then normalized to the β-actin (goat anti-porcine β-actin, Adlitteram Diagnostic Laboratories, USA). This assay was performed in duplicate for each sample.
The body weight and liver weight of newborn NBW and IUGR piglets are shown in Table 1. The results indicated that the piglets with IUGR had significantly lower birth weight (p<0.01), liver weight (p<0.05) and relative liver weight (p<0.05) than piglets with NBW.
ALT and AST activities in the serum of NBW and IUGR newborn piglets are presented in Table 2. The activity of ALT in the serum of IUGR piglets was significantly higher (p<0.01) than in NBW piglets. Meanwhile, serum AST activity was also increased (p<0.05) in IUGR piglets compared with normal counterparts.
The activities of T-SOD, GPx, CAT and content of MDA in the liver of piglets are shown in Table 3. The T-SOD and GPx activities were significantly lower in IUGR piglets (p<0.01); but there was no statistical differences in hepatic CAT activity between IUGR and NBW. In addition, the MDA generation in the liver of NBW piglets was significantly lower (p<0.05) than in IUGR piglets. Taken together, these results suggested that IUGR affected the antioxidant system by inducing lipid peroxidation and reducing antioxidant capacity.
Representative photomicrographs of liver sections from NBW and IUGR group are shown in Figure 1. Histological changes of the livers are illustrated in Figure 1a and Figure 1b. In the NBW sections, tissue morphology was normal, whereas IUGR piglets had moderate alteration in hepatic morphology, including the presence of fatty hepatic infiltrates and cytoplasmic vacuolization in the midzonal and/or entire hepatic lobule, although large necrotic foci was not observed. Results from the immunohistochemical analyses revealed that Hsp70 was expressed in the liver of NBW and IUGR piglets, and the signal was distributed diffusely in both the nucleus and cytoplasm of hepatocytes (Figure 1d). Further, a higher intensity of staining was presented in the midzonal hepatic lobule (Figure 1c), namely liver acinar zone three. In Figure 1e, there is lack of Hsp70 immunoreactivity when antibody was omitted.
The expression data for Hsp70 protein and Hsp70 protein normalized to β-actin are shown in Table 3. The ELISA results demonstrated that IUGR piglets had 15.0% (p<0.05) higher normalized Hsp70 protein in liver in comparison to NBW piglets.
Hsp70, as the best-known member of Hsp family, is present in cytosol and subcellular compartments (e.g. nucleus, mitochondria) of various cell types from prokaryotes to eukaryotes. This ubiquitous and highly conserved protein can be induced by both physiological perturbations (e.g. fever, bacterial and viral infections, inflammation, ischemia and tumors) and stress factors (e.g. oxidative stress, toxic chemicals, heavy metals, nutritional deficiencies), followed by enhanced cytoprotective ability to increase the survival rate of stressed cells (Bedirli et al., 2004; Otaka et al., 2006; Zhang et al., 2006; Li et al., 2007; Padmini et al., 2009). The results of this study showed that the liver structure and function were impaired in neonate IUGR piglets, accompanied by altered redox state. In addition, increased expression of hepatic Hsp70 protein was seen in IUGR piglets.
Compared with normal offspring, neonatal infants with IUGR not only have the lower birth weight and smaller organs but also suffer from dysfunction in several systems, especially in the digestive system (Wang et al., 2005; Lipsett et al., 2006). Previous studies confirmed that, in addition to reduced volume and size (Latini et al., 2004), the liver of IUGR infant usually has elevated neonatal cholestasis, diminished urea synthesis, decreased capacity for amino acid utilization and decreased activities of hepatic microsomal monooxygenases (Boehm et al., 1990). The results of this study also showed significantly lower liver weight of newborn IUGR piglets with increased serum ALT and AST activities. These two aminotransferases are interpreted as an index of hepatic injury and their release into the blood indicates the hepatic pathological damage (Zhang et al., 2006; Inoue et al., 2008). Furthermore, the liver of IUGR piglets suffered from histological impairment in this study, including steatosis and cytoplasmic vacuolization. The impairment was observed in the entire hepatic lobule but occurs more readily in the midzonal hepatic lobule. Thus, our data indicated that IUGR led to hepatic injury in newborn piglets. The abnormal liver of IUGR infant is usually associated with either sparing or sacrifice of fetal liver growth under the condition of placental insufficiency (Gentili et al., 2009). Uteroplacental insufficiency, which is the common cause of IUGR, limits the supply of critical substrates such as oxygen and glucose to the fetus. Preferential blood flowed to the brain and heart may furthermore deprive other organs of oxygen and macro- and micronutrients (Latini et al., 2004; Simmons et al., 2005). A major consequence of limited nutrient availability is an alteration in the redox state in susceptible fetal tissues (Simmons, 2006), such as midzonal hepatic lobule, leading to oxidative stress, structural and functional derangement and eventually cell death (Bedirli et al., 2004). This is in agreement with our finding in the antioxidant system of IUGR piglet liver. Taken together, it appears that IUGR piglet failed to utilize antioxidant defense system to prevent oxidative stress and oxidative injury in liver.
A substantial amount of studies showed that IUGR can change the expression of critical proteins either at transcriptional and/or post-transcriptional levels, resulting in the functional alterations of organs or tissues (Lane et al., 2002; Baserga et al., 2005; Wang et al., 2008; Gentili et al., 2009), but there were limited studies on Hsp70 expression in IUGR offspring. Liu et al. (2008) reported that with the placental vascular disease induced by IUGR, the expression of Hsp70 was upregulated remarkably in placental tissue. The increase of Hsp70 expression was also observed in the intestine of neonatal and adult IUGR pigs (Zhong et al., 2010; Wang et al., 2012). In agreement with these results, our semiquantitative detection of Hsp70 demonstrated that the expression of Hsp70 protein was higher in the liver of IUGR piglets. Meanwhile, the immunohistochemical staining shown that increased amount of Hsp70 was observed in the area where the liver injury occurs in IUGR piglet. These results suggested that increased Hsp70 protein expression might be associated with the hepatic oxidative injury. Several previous studies have also indicated the interrelationship between Hsp70 expression and redox state in organs (King et al., 2002; Zhang et al., 2006; Padmini et al., 2009). As heat-shock factor-1 (HSF-1), which controls the transcription of Hsp70, is redox-regulated (Ahn and Thiele, 2003), the expression of Hsp70 is predominantly dependent on the cellular redox state and strongly induced by oxidative stress (Kalmar and Greensmith, 2009). On the other hand, it is known that the hepatocytes in different zones have quantitative differences in structural characteristics and enzyme levels (Yan et al., 2008). The hepatocytes in zone three, where the oxygen concentration is the poorest among zones, are susceptible to damage caused by hypoxia and lipid peroxidation (MacDonald et al., 2001). Thus, this might be the reason why Hsp70 protein predominantly accumulates in zone three when oxidative stress occurs (Schwimmer et al., 2005; Savransky et al., 2007). Thus, it is reasonable to conclude that the overexpression of Hsp70 protein could predominantly accumulate in zone three when stress occurs in liver.
Overexpression of Hsp70 may in turn contribute to the decrease of reactive oxygen species accumulation and thus confer protection against oxidative stress-induced injury. During ischemic stress, Hsp70 increased the activities of GPx and glutathione reductase, which were important members of GSH-related antioxidant enzymes (Guo et al., 2007). Furthermore, the increase of mitochondrial Hsp70 is attributed to the prevention of NO-dependent increase in cellular free iron thereby maintaining the Fe-S clusters for the intactness and integrity of mitochondrial respiratory chain complexes (Padmini et al., 2009). The increased mitochondrial Hsp70 prevents the collapse of the mitochondrial membrane potential and the release of cytochrome c from mitochondria and hence reduces free redical damage during oxidative stress (Chiu et al., 2009). However, we haven’t observed the improved redox state in response to the increase of Hsp70 expression in the liver of IUGR piglets. Perhaps, Hsp70 is not adequate to completely eliminate the hepatic oxidant stress. It is reported that critical features of IUGR fetuses such as hypoxia and hypoglycaemia usually normalized within days after birth in the human (De Prins and Van Assche, 1982), so we speculated that the redox homeostasis might also be rebuilt later and Hsp70 might play a role in this process, which needs further investigation.
In conclusion, this present study provides the first description of an increased Hsp70 expression in the liver of neonatal IUGR piglets, which may be partially caused by oxidative injury. The results indicated that IUGR offspring suffered from oxidant stress in the liver at birth.
ACKNOWLEDGEMENT
This study was financially supported by a grant from the National Natural Science Foundation of China (Project No.3097216). We are grateful to Dr L.J. (Kelvin) Koong for critical discussions and improving the English language of the manuscript. We also thank Gaiqin Wang and other colleagues for their assistance during the research.
Figure 1
Histology of the liver and Hsp70 protein distribution in the liver. (a) Normal lobular architecture and cell structure in the liver of NBW piglets; (b) IUGR newborn piglets had alterations in hepatic morphology, including the presence of fatty hepatic infiltrates, cytoplasmic vacuolization and sinusoidal congestion. HE staining, 400×. (c), (d) Hsp70 was distributed diffusely both in the nucleus and cytoplasm of hepatocytes (arrows), and the positive signal was more pronounced in the zone three of liver acinus of IUGR piglets; (e) Absence of Hsp70 staining when antibody was omitted. IHC staining, 200× in c, 400× in d and e.
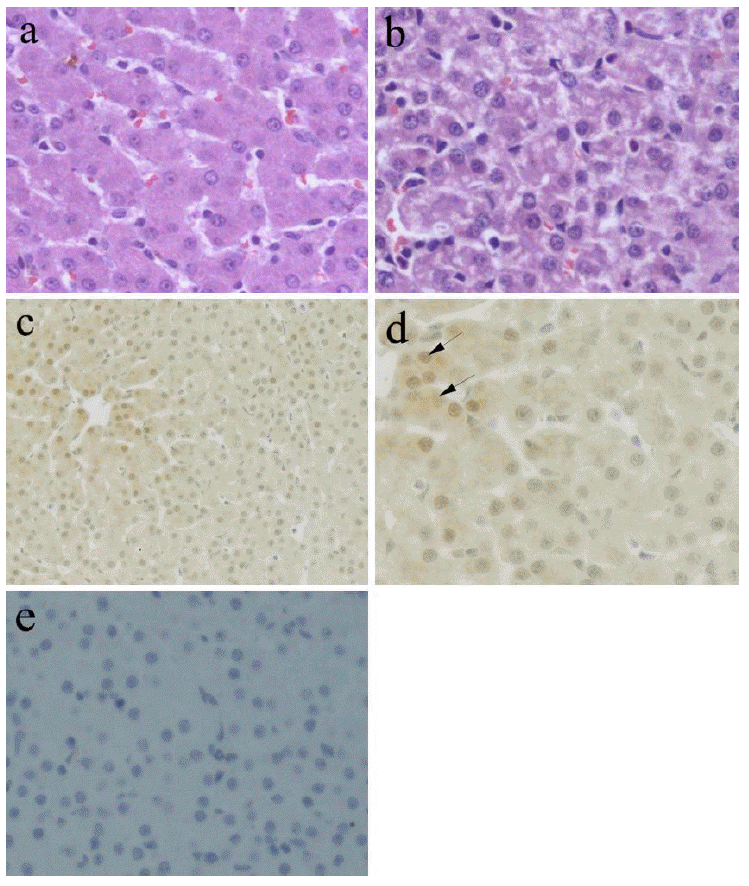
Table 1
Body and liver weight of newborn piglets with NBW and with IUGR
| Items | NBW (n = 5) | IUGR (n = 5) |
|---|---|---|
| Body weight (kg) | 1.48±0.10 | 0.76±0.04** |
| Liver weight (g) | 45.93±8.26 | 18.61±2.48* |
| Liver weight/body weight (%) | 3.13±0.01 | 2.46±0.01* |
Table 2
Effect of IUGR on the activities of ALT and AST in the serum of piglets
| Items | NBW (n = 5) | IUGR (n = 5) |
|---|---|---|
| ALT (U/L) | 10.39±2.40 | 27.71±2.84** |
| AST (U/L) | 21.78±4.36 | 35.79±4.30* |
Table 3
Effect of IUGR on the antioxidant ability and Hsp70 expression in the liver of piglets
| Items | NBW (n = 5) | IUGR (n = 5) |
|---|---|---|
| T-SOD (U/mg protein) | 100.5±9.0 | 28.3±9.7** |
| GPx (U/mg protein) | 111.3±4.7 | 82.3±7.3** |
| CAT (U/mg protein) | 74.1±9.5 | 57.5±8.7 |
| MDA (nmol/mg protein) | 3.26±1.32 | 6.88±1.66* |
| Hsp70 (μg/g tissue) | 0.14±0.01 | 0.17±0.02 |
| Hsp70 (μg/g protein) | 18.90±5.64 | 21.95±7.84 |
| Hsp70/β-actin | 0.57±0.04 | 0.65±0.03* |
REFERENCES
Ahn SG, Thiele DJ. 2003. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev 17:516–528.



Aucott SW, Donohue PK, Northington FJ. 2004. Increased morbidity in severe early intrauterine growth restriction. J Perinatol 24:435–440.


Baserga M, Hale MA, McKnight RA, Yu X, Callaway CW, Lane RH. 2005. Uteroplacental insufficiency alters hepatic expression, phosphorylation, and activity of the glucocorticoid receptor in fetal IUGR rats. Am J Physiol Regul Integr Comp Physiol 289:R1348–R1353.


Bedirli A, Sakrak O, Muhtaroglu S, Soyuer I, Guler I, Riza Erdogan A, Sozuer EM. 2004. Ergothioneine pretreatment protects the liver from ischemia-reperfusion injury caused by increasing hepatic heat shock protein 70. J Surg Res 122:96–102.


Boehm G, Müller DM, Teichmann B, Krumbiegel P. 1990. Influence of intrauterine growth retardation on parameters of liver function in low birth weight infants. Eur J Pediatr 149:396–398.


Chiu HY, Tsao LY, Yang RC. 2009. Heat-shock response protects peripheral blood mononuclear cells (PBMCs) from hydrogen peroxide-induced mitochondrial disturbance. Cell Stress Chaperones 14:207–217.



De Prins FA, Van Assche FA. 1982. Intrauterine growth retardation and development of endocrine pancreas in the experimental rat. Neonatology 41:16–21.

Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. 1995. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol Regul Integr Comp Physiol 268:R28–R32.

Gentili S, Morrison JL, McMillen IC. 2009. Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol Reprod 80:1121–1127.



Guo S, Wharton W, Moseley P, Shi H. 2007. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 12:245–254.



Inoue K, Matsumoto M, Miyoshi Y, Kobayashi Y. 2008. Elevated liver enzymes in women with a family history of diabetes. Diabetes Res Clin Pract 79:e4–e7.


Kalmar B, Greensmith L. 2009. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev 61:310–318.


Kanemura H, Kusumoto K, Miyake H, Tashiro S, Rokutan K, Shimada M. 2009. Geranylgeranylacetone prevents acute liver damage after massive hepatectomy in rats through suppression of a CXC Chemokine GRO1 and induction of heat shock proteins. J Gastrointest Surg 13:66–73.


King YT, Lin CS, Lin JH, Lee WC. 2002. Whole-body hyperthermia-induced thermotolerance is associated with the induction of heat shock protein 70 in mice. J Exp Biol 205:273–278.



Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD. 2002. Increased hepatic peroxisome proliferator-activated receptor-γ coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology 143:2486–2494.


Latini G, De Mitri B, Del Vecchio A, Chitano G, De Felice C, Zetterstrom R. 2004. Foetal growth of kidneys, liver and spleen in intrauterine growth restriction: “programming” causing “metabolic syndrome” in adult age. Acta Paediatr 93:1635–1639.


Li H, Sui C, Kong F, Zhang H, Liu J, Dong M. 2007. Expression of HSP70 and JNK-related proteins in human liver cancer: Potential effects on clinical outcome. Dig Liver Dis 39:663–670.


Lipsett J, Tamblyn M, Madigan K, Roberts P, Cool JC, Runciman SI, McMillen IC, Robinson J, Owens JA. 2006. Restricted fetal growth and lung development: A morphometric analysis of pulmonary structure. Pediatr Pulmonol 41:1138–1145.


Liu Y, Li N, You L, Liu X, Li H, Wang X. 2008. HSP70 is associated with endothelial activation in placental vascular diseases. Mol Med 14:561–566.



MacDonald GA, Bridle KR, Ward PJ, Walker NI, Houglum K, George DK, Smith JL, Powell LW, Crawford DH, Ramm GA. 2001. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol 16:599–606.


Otaka M, Odashima M, Watanabe S. 2006. Role of heat shock proteins (molecular chaperones) in intestinal mucosal protection. Biochem Biophys Res Commun 348:1–5.


Padmini E, Vijaya Geetha B, Usha Rani M. 2009. Pollution induced nitrative stress and heat shock protein 70 overexpression in fish liver mitochondria. Sci Total Environ 407:1307–1317.


Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. 2007. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293:G871–G877.


Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. 2005. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 42:641–649.


Simmons RA. 2006. Developmental origins of diabetes: the role of oxidative stress. Free Radic Biol Med 40:917–922.


Simmons RA, Suponitsky-Kroyter I, Selak MA. 2005. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to β-cell failure. J Biol Chem 280:28785–28791.


Trahair JF, DeBarro TM, Robinson JS, Owens JA. 1997. Restriction of nutrition in utero selectively inhibits gastrointestinal growth in fetal sheep. J Nutr 127:637–641.


Wang J, Chen L, Li D, Yin Y, Wang X, Li P, Dangott LJ, Hu W, Wu G. 2008. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr 138:60–66.


Wang T, Huo Y, Shi F, Xu R, Hutz R. 2005. Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate 88:66–72.


Wang Y, Zhang L, Zhou G, Liao Z, Ahmad H, Liu W, Wang T. 2012. Dietary l-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br J Nutr 1:1–11.

Wataba K, Saito T, Takeuchi M, Nakayama M, Suehara N, Kudo R. 2004. Changed expression of heat shock proteins in various pathological findings in placentas with intrauterine fetal growth restriction. Med Electron Microsc 37:170–176.


Yan L, Ropella GP, Park S, Roberts MS, Hunt CA. 2008. Modeling and simulation of hepatic drug disposition using a physiologically based, multi-agent in silico liver. Pharm Res 25:1023–1036.


Zadrona M, Gawlik M, Nowak B, Marcinek A, Mrowiec H, Walas S, R Wietecha-Posuszny, P Zagrodzki. 2009. Antioxidants activities and concentration of selenium, zinc and copper in preterm and IUGR human placentas. J Trace Elem Med Biol 23:144–148.


Zhang HJ, Doctrow SR, Oberley LW, Kregel KC. 2006. Chronic antioxidant enzyme mimetic treatment differentially modulates hyperthermia-induced liver HSP70 expression with aging. J Appl Physiol 100:1385–1391.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





