 |
 |
| Anim Biosci > Volume 34(8); 2021 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
ACKNOWLEDGMENTS
Figure 1

Figure 2
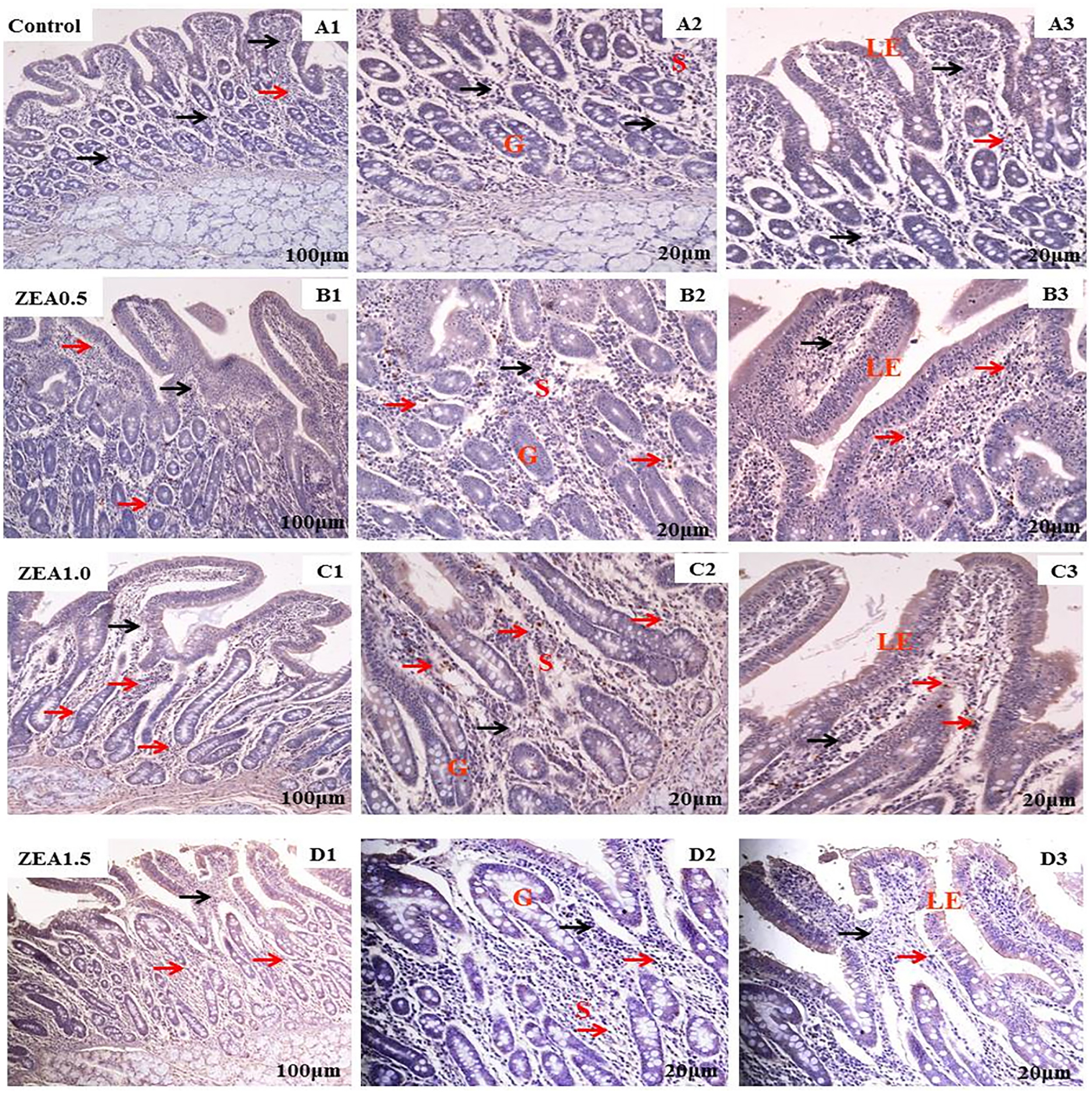
Figure 3
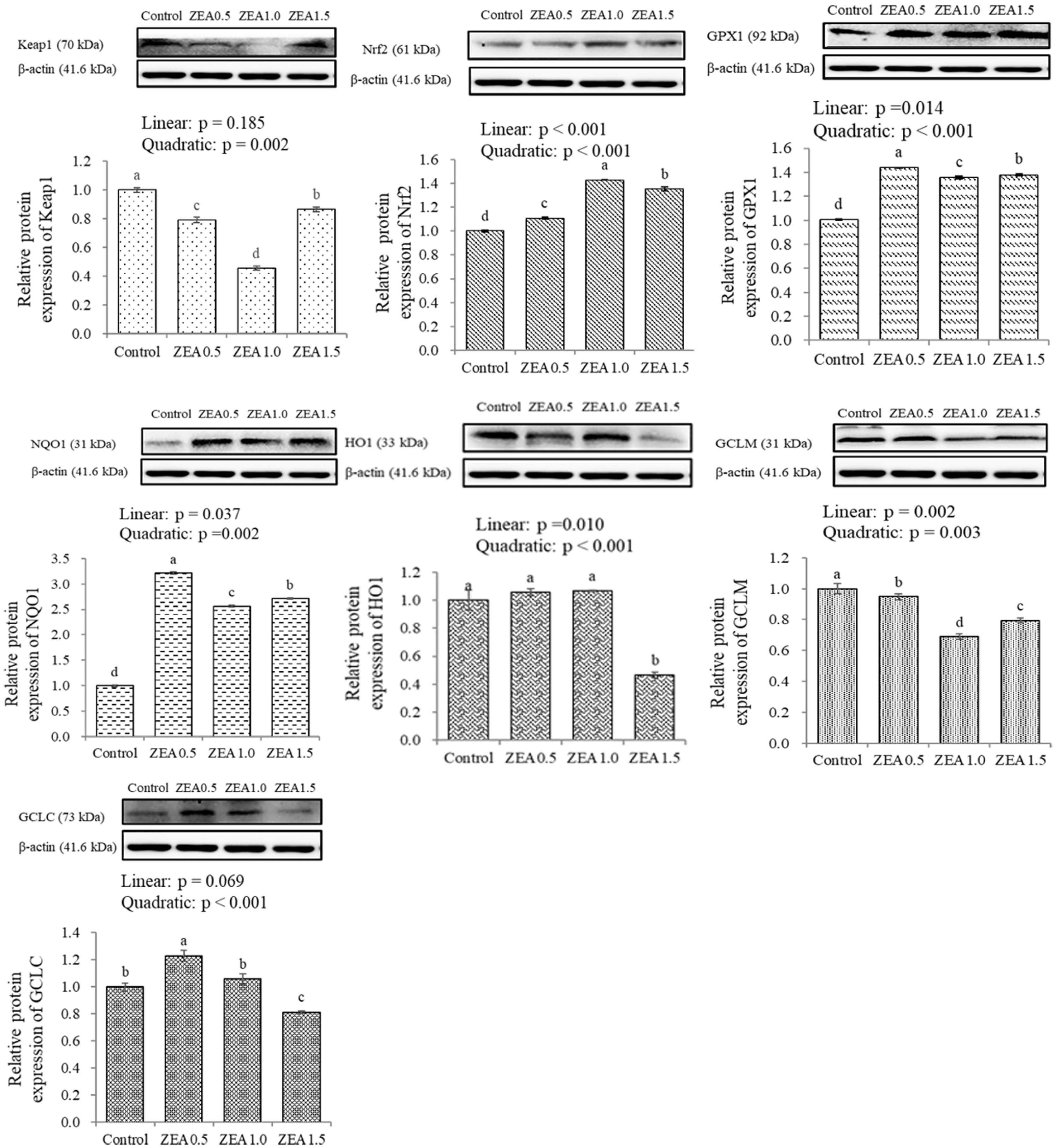
Table 1
| Items | Composition |
|---|---|
| Ingredients (%) | |
| Corn | 64.5 |
| Whey powder | 5.0 |
| Soybean meal | 23.0 |
| Fish meal | 5.0 |
| L-lysine HCl | 0.2 |
| CaHPO4 | 0.7 |
| Pulverized limestone | 0.3 |
| NaCl | 0.3 |
| Premix1) | 1.0 |
| Nutrients2) | |
| Digestible energy (MJ/kg) | 13.81 |
| Crude protein (%) | 19.82 |
| Calcium (%) | 0.70 |
| Total phosphorus (%) | 0.64 |
| Lysine (%) | 1.22 |
| Sulfur amino acid (%) | 0.65 |
| Threonine (%) | 0.75 |
| Trptophan (%) | 0.22 |
1) Premix provided the following per kilogram of diet: 3,300 IU vitamin A, 330 IU vitamin D3, 24 IU vitamin E, 0.75 mg vitamin K3, 1.50 mg vitamin B1, 5.25 mg vitamin B2, 0.02625 mg vitamin B12, 15.00 mg pantothenate, 22.50 mg niacin, 0.075 mg biotin, 0.45 mg folic acid, 6.00 mg Mn, 150 mg Fe, 150 mg Zn, 9.00 mg Cu, 0.21 mg I, and 0.45 mg Se.
2) Digestible energy was obtained from digestion experiment [25], whereas the other nutrient contents were calculated values.
Table 2
| Target gene | Accession No. | Primer Sequence (5′ to 3′)1) | Product size (bp) |
|---|---|---|---|
| Nrf2 | RA_011763 |
F: GAAAGCCCAGTCTTCATTGC R: TTGGAACCGTGCTAGTCTCA |
190 |
| GPX1 | NC_010444.3 |
F: GGCACAACGGTGCGGGACTA R: AGGCGAAGAGCGGGTGAGCA |
159 |
| Keap1 | NM_001114671.1 |
F: GCCTCATCGAGTTCGCTTAC R: CACGGACCACACTGTCAATC |
105 |
| HO1 | NM_001004027.1 |
F: GCTGAGAATGCCGAGTTCAT R: TGTAGACCGGGTTCTCCTTG |
142 |
| NQO1 | NM_ 001159613.1 |
F: TGAATTACATCTCTGTGGTTTA R: AGAATGACACTCATATTAGGCG |
171 |
| GCLM | CV868255.1 |
F: ACAATACAACGGTTCAGGTGAGT R: GCCTGTAAAATGTGTCATTGAGG |
122 |
| GCLC | CV864761.1 |
F: ATGGGCTGGGAACAAGATGT R: GTAACAGGTCTGCATCCTCATC |
119 |
| GAPDH | NM_001206359.1 |
F: ATGGTGAAGGTCGGAGTGAA R:CGTGGGTGGAATCATACTGG |
154 |
Nrf2, nuclear factor erythroid 2-related factor 2; Keap1, Kelch-like ECH-associated protein1; GPX1, glutathione peroxidase 1; HO1, hemeoxygenase 1; NQO1, quinone oxidoreductase 1; GCLM, modifier subunit of glutamate-cysteine ligase; GCLC, catalytic subunit of glutamate-cysteine ligase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Table 3
| Items | Control1) | ZEA0.51) | ZEA1.01) | ZEA1.51) | SEM | p-values | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Treatment | Linear | Quadratic | ||||||
| T-SOD (U/mg prot) | 19.30a | 13.14b | 12.05c | 11.37d | 0.109 | <0.001 | <0.001 | <0.001 |
| GSH-Px (U/mL) | 165.89a | 136.53b | 122.73c | 112.29d | 0.845 | <0.001 | <0.001 | <0.001 |
| MDA (nmol/mg prot) | 4.62c | 4.55c | 6.54a | 4.99b | 0.048 | <0.001 | 0.106 | 0.045 |
SEM, standard error of the mean; T-SOD, total superoxide dismutase; GSH-PX, glutathione peroxidase; MDA, malondialdehyde.
Table 4
| Items | Control1) | ZEA0.51) | ZEA1.01) | ZEA1.51) | SEM | p-values | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Treatment | Linear | Quadratic | ||||||
| Nrf2 | 0.46b | 0.97a | 0.92a | 0.97a | 0.019 | <0.001 | <0.001 | <0.001 |
| GPX1 | 0.35b | 0.67a | 0.71a | 0.67a | 0.008 | <0.001 | <0.001 | <0.001 |
SEM, standard error of the mean; Nrf2, nuclear factor erythroid 2-related factor 2; GPX1, glutathione peroxidase 1.
Table 5
| Items | Control1) | ZEA0.51) | ZEA1.01) | ZEA1.51) | SEM | p-values | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Treatment | Linear | Quadratic | ||||||
| Keap1 | 1.01a | 1.10a | 0.63b | 0.26c | 0.018 | <0.001 | 0.022 | 0.005 |
| Nrf2 | 1.03b | 1.14a | 1.14a | 1.14a | 0.004 | <0.001 | <0.001 | <0.001 |
| GPX1 | 1.08c | 1.21b | 1.39a | 1.39a | 0.014 | <0.001 | <0.001 | <0.001 |
| NQO1 | 1.00b | 1.16a | 0.25c | 0.19c | 0.022 | <0.001 | <0.001 | <0.001 |
| HO1 | 1.00b | 0.44c | 1.67a | 0.26c | 0.034 | <0.001 | 0.349 | 0.128 |
| GCLM | 1.01c | 3.19a | 1.46b | 1.52b | 0.067 | <0.001 | 0.903 | 0.010 |
| GCLC | 1.01a | 0.99a | 0.55b | 0.24c | 0.030 | <0.001 | <0.001 | <0.001 |
SEM, standard error of the mean; Keap1, Kelch-like ECH-associated protein1; Nrf2, nuclear factor erythroid 2-related factor 2; GPX1, glutathione peroxidase 1; NQO1, quinone oxidoreductase 1; HO1, hemeoxygenase 1; GCLM, modifier subunit of glutamate-cysteine ligase; GCLC, catalytic subunit of glutamate-cysteine ligase.
REFERENCES
- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





