Effect of Sea Tangle (Laminaria japonica) and Charcoal Supplementation as Alternatives to Antibiotics on Growth Performance and Meat Quality of Ducks
Article information
Abstract
A total of 150 growing ducks were assigned to five dietary treatments to study the effect of sea tangle and charcoal (STC) supplementation on growth performance and meat characteristics in a completely randomized design. There were six replicates and five ducklings in each replication. The five dietary treatments were control, antibiotic, and 0.1%, 0.5%, and 1% STC supplemented diets. No significant differences were found on ADG, ADFI, and gain:feed among treatments in different weeks. The overall (0 to 3 weeks) ADFI decreased in antibiotic treatment (p<0.05) whereas the gain:feed increased significantly upon 1.0% STC supplementation compared to control (p<0.05). No significant variation was found in meat chemical composition except crude fat content which was high in 1.0% STC dietary group (p<0.05). Meat cholesterol was reduced in 0.1% STC group (p<0.05) compared to other dose levels while serum cholesterol was unaffected. High density lipoprotein (HDL) content was high in 1.0% STC (p<0.05) and low density lipoprotein (LDL) was low in 0.1% and 1.0% STC dietary groups (p = 0.06). No significant effect was found on the thiobarbituric acid reactive substances (TBARS) of fresh meat, whereas the TBARS value of meat preserved for 1 week was reduced significantly in STC dietary groups (p<0.05). The 0.1% STC dietary group showed an increased myristic acid (p = 0.07) content whereas, the content of eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids increased in STC supplementation than antibiotic group (p<0.05). An increased concentration of omega-3 fatty acids and a reduced ratio of n-6/n-3 PUFA ratio was found upon 1.0% STC supplementation compared to antibiotic dietary group (p<0.05). Therefore, 1.0% STC dietary supplementation can be used as alternatives to antibiotics in duck production.
INTRODUCTION
Antibiotics that are often used to treat illnesses in humans are also added to animal diets, particularly in the poultry industry. Antibiotic supplementation at the sub-therapeutic level to the poultry diet is common as it reduces the incidence of disease and improves growth rate, feed efficiency, and meat quality. Furthermore, the frequent use of antibiotics to increase growth also compensates for overcrowded and unsanitary conditions. In 2011, more antibiotics were sold for use in meat and poultry production than ever before (Pew Campaign on Human Health and Industrial Farming, 2013). Although people enjoy the benefits from antibiotics used in animal production, the extensive use of antibiotics as therapeutics and growth promoters could lead to problems such as antibiotic residues and increased bacterial resistance. Thus, alternative sources of antibiotic with equal efficacy need to be evaluated (Bae et al., 1999).
Farmers worldwide use different types of unconventional feed resources as feed additives on the basis of their availability and economical consideration. Sea tangle (Laminaria japonica) is an edible brown seaweed that is a popular dietary supplement and traditional marine foodstuff in Korea (Kim et al., 2005; Athukorala et al., 2007). Sea tangle is enriched in protein, amino acids, minerals, polyphenols, and dietary fiber (Ahn et al., 2010) and displays several biological activities, such as antioxidant activity, anti-mutagenic activity, and antibacterial activity (Okai et al., 1993; Wang et al., 2006; Park et al., 2009). Seaweed contains many different types of polysaccharides having chemical structures related to the corresponding taxonomic classification of algae and their cell structures (Ferreira et al., 2012; Wijesinghe et al., 2012). Polysaccharides can act as prebiotics (substances that stimulate the growth of beneficial bacteria in the digestive tract) and exert growth-promoting as well as health-improving effects (Vidanarachchi et al., 2009). Many are soluble dietary fibers and have positive effects in the digestive tracts of animals (i.e. alginic acid). Asar (1972) reported that supplementation of 4% seaweeds in chicken diet increased body weight gain whereas Tomova et al. (1980) reported no adverse effect of supplementation 3% and 4% seaweed meal in broiler diet on FCR and slaughter traits. Maurice et al. (1984) concluded that sun dried Brazilian elodea can be used in broiler diets at 5% without adversely affecting growth, FCR or dressing percentage. Gu et al. (1988) concluded that 2% of marine algae meal improved broiler performance and dressing percentage. Seaweed can be used in starter and finisher duck diets up to 12% and 15% without adversely affecting growth performance and carcass quality (EI-Deek and Brikaa, 2009). It is well known that activated charcoal, obtained by treating charcoal with chemicals, is capable of binding to toxins, intestinal gases, and fat due to its highly absorbent structural characteristics (Blacow, 1972). Activated charcoal is a universal adsorbent as it can bind with a variety of molecules (Chandy and Sharma, 1998). Dietary inclusion of wood charcoal in broiler diet improved broiler performance (Kutlu and Ü nsal, 1998). Therefore, the objective of this study was to evaluate the potential of sea tangle and charcoal (STC) supplementation as alternatives to antibiotics in ducks in order to meet the demands of the poultry industry in countries where excessive amounts of antibiotics are used as growth promoters.
MATERIALS AND METHODS
Birds, diets, and experimental design
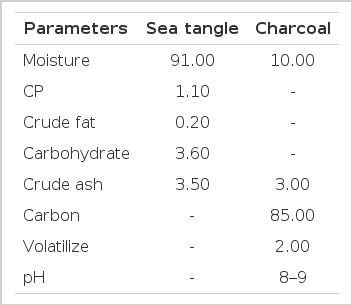
A total of 150 growing ducks (Cherry berry, SUPER M3 F1; 22 days old) were used for a period of 3 weeks. The ducklings were assigned to five dietary treatments with six replications of five ducklings in each following a completely randomized design. Sea tangle (edible powder) and charcoal (livestock feed grade) were purchased from Suncheon, South Korea. Charcoal was ground and mixed with sea tangle (1:1) and used at the levels of 0.1%, 0.5%, and 1.0% with the basal diet. There were five dietary treatment groups: Control (basal diet), antibiotic (basal diet +0.01% Chlortetracycline), 0.1% STC (basal diet+0.1% STC), 0.5% STC (basal diet+0.5% STC), and 1.0% STC (basal diet+1.0% STC). The composition of sea tangle and wood charcoal are represented in Table 1. All diets were formulated to meet or exceed the nutrient requirements of growing ducks (NRC, 1994). The ingredients and chemical composition of the diets are shown in Table 2.
Measurements and analysis
Growth performance
Body weights were measured on a weekly basis from the beginning to end of the experiment. Feed intake was determined by measuring feed residue on a weekly basis from the start of the experiment. Gain:feed was obtained dividing the body weight gain by feed intake.
Duck breast muscle composition
At the end of the experiment, ducklings were slaughtered and breast muscle samples were collected. Moisture, CP, crude fat, and ash percentage of meat samples were analyzed according to Association of Analytical Communities (AOAC, 2000) methods. The cholesterol content of breast meat was determined by gas chromatography (DS 6200, Donam, South Korea), according to the method described by Yang et al. (2003).
Serum biochemical parameters and oxidative stability of duck meat
Blood samples were obtained at the end of the experiment by wing puncture and centrifuged at 3,000 rpm for 20 min. Serum was collected and then analyzed for total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) concentrations by using a blood analyzer (COBAS MIRA; Roche, Mannheim, Germany). To determine the oxidative stability of duck meat, breast meat samples were preserved in the refrigerator at 4.5°C, and thiobarbituric acid reactive substances (TBARS) of meat were assayed for fresh meat as well as on day 7 according to the method of Sarker and Yang (2011). TBARS values were expressed as micromoles of malondialdehyde (MDA) per 100 g of meat sample.
Fatty acid composition
Fatty acid composition was determined from breast meat by the methyl ester extraction method according to Yang et al. (2003). Fatty acids were identified by matching their retention times with those of their relative standards (PUFA-2, Animal Source, SUPELCO, Bellefonte, PA, USA) as well as by following the Food Composition Table (NRLSI, 2002).
Statistical analysis
Data were analyzed using the general linear models of SAS Institute (2003) to estimate variance components with a completely randomized design. Orthogonal contrasts were used to study the dietary effects and treatment means were computed with the LSMEANS option. Variability in the data were expressed as SE, and a probability level of p<0.05 was considered as statistically significant, whereas a trend was expressed when p<0.10.
RESULTS
Growth performance
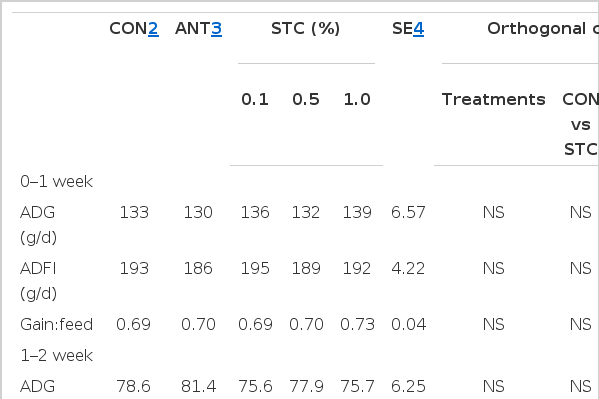
ADG, ADFI, and gain:feed were considered as growth parameters of duck in this experiment. The performances of growing ducks are shown in Table 3. Results showed that there was no significant variation in ADG, ADFI, or gain:feed of duck meat on weekly basis. The ADFI of ducks was significantly reduced in antibiotic treatment group compared to control (p = 0.08) and STC dietary groups (p<0.05). The gain:feed of ducks were significantly increased in antibiotic group compared to control dietary group (p<0.05) while it was also significantly increased in STC supplementation particularly in 1.0% STC dietary group (p = 0.07) compared with the control.
Duck breast muscle composition
The chemical compositions of duck breast muscle are represented in Table 4. There was no significant variation in moisture, crude ash and CP content of duck meat among the treatment groups. Although there was no significant effect of STC dietary groups on crude fat content of duck meat compared to control or antibiotic, a significant difference was observed within STC dietary groups which was most pronounced in the 1.0% STC dietary groups (p<0.05). The cholesterol concentration of duck meat remained unaffected among the treatments, except for the 1.0% STC dietary supplementation where it was significantly reduced (p<0.05).
Serum biochemical parameters and oxidative stability of duck meat
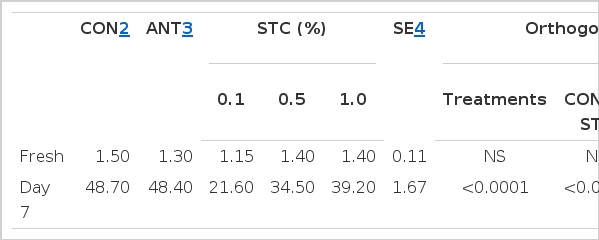
Serum cholesterol, HDL, and LDL concentrations are shown in Table 5. There was no significant change in the total cholesterol concentration of duck serum among the dietary groups. There was an increasing trend of HDL concentration with an increasing rate of STC supplementation and was significantly higher (p<0.05) in 1.0% STC group compared to 0.1% STC group. The highest LDL concentration observed in 0.5% STC dietary group which was significantly different from the 0.1% and 1.0% STC dietary groups (p = 0.06). The oxidative stability of duck meat is represented in the Table 6. The TBARS value of fresh duck meat did not show any significant variation among the treatment groups although the 0.1% STC dietary group displayed a lower tendency (p = 0.08). However, after 1 week of preservation at 4°C, the highest TBARS values were observed in the control and antibiotic groups, whereas the STC dietary groups showed a reduced TBARS value, particularly in the 0.1% STC dietary group (p<0.05).
Fatty acid composition
Total fatty acid compositions of fresh duck meat are presented in Table 7. Among individual fatty acids, myristic acid content increased significantly (p = 0.06) in 0.1% STC supplementation compared to control. Considering individual polyunsaturated fatty acids, increased linoleic acid content was found in both 0.5% and 1.0% STC dietary groups compared to 0.1% STC supplementation. The eicosadienoic acid content was significantly increased (p<0.05) by 0.1% and 0.5% STC dietary supplementation compared to antibiotic treatment whereas it did not differ from the control group. The eicosapentaenoic acid (EPA) content of duck meat significantly decreased (p = 0.0001) in all dietary groups compared to control. Among the STC supplemented group 1.0% STC showed a significant increase (p<0.05) of EPA content compared to antibiotic and other dose levels (p<0.05). Supplementation of 0.5% STC significantly increased the docosahexaenoic acid content of duck meat compared to control (p<0.05) whereas, it did not differ with antibiotic group. There was no variation in the total concentration of saturated fatty acid (SFA), unsaturated fatty acid (UFA) and monounsaturated fatty acid (MUFA) among the treatments. The polyunsaturated fatty acids (PUFA) showed an increasing tendency in 0.5% and 1.0% STC dietary groups (p = 0.08) compared to 0.1% STC supplementation. The UFA/SFA ratio increased significantly (p = 0.08) in STC dietary group than control group particularly in 1.0% STC dietary group. The MUFA/SFA ratio also significantly increased upon antibiotic and STC supplementation (p = 0.05) compared with the control dietary group. The concentration of omega-3 fatty acids was elevated in the STC dietary supplementation compared to the antibiotic group (p<0.05) particularly rich in 1.0% STC dietary group whereas the lowest content of omega-6 fatty acid was found in 0.1% STC supplementation. A reduced ratio of n-6/n-3 PUFA ratio was observed (p<0.05) upon STC supplementation which was also pronounced in 1.0% STC dietary groups (p<0.05).
DISCUSSION
The study was conducted to evaluate the combined effects of STC supplementation on the growth performance, meat characteristics, and fatty acid composition of growing duck in comparison to antibiotic use. Considering individual weeks, there was no significant variation in ADG, ADFI, or gain:feed between antibiotic and STC dietary groups, which could be due to the combined effect of STC supplementation. You et al. (2009) reported that consumption of sea tangle powder reduced average body weight in Korean female college students. In contrast, Kutlu and Ünsal (1998) reported that supplementation of wood charcoal improved feed efficiency and body weight gain in broilers. The improvement in gain:feed and reduction of ADFI during overall (0 to 3 week) period in growing ducks by STC supplementation also was supported by the findings of Kutlu and Ünsal (1998) and Vidanarachchi et al. (2009).
Although higher than 76% moisture in meat was observed in the treatment groups, there was no significant variation in CP content, which supports the results of Leonard (2010) that seaweed contains higher moisture with a protein content less than 5%. Aguilera-Morales et al. (2005) reported that most algae species contain all of the essential amino acids for vertebrates at higher levels than an equivalent weight of soybeans. In this study, control and antibiotic diets did not have any extra effect on duck meat composition. Burtin (2003) and Polat and Ozogul (2008) reported the lipid content of seaweeds ranges from 1% to 5% of dry weight. Kutlu and Ünsal (1998) reported that supplementation of charcoal to the diets tended to reduce abdominal fat weight and abdominal fat percentage, while having no significant effects on carcass yield. The crude fat content of different dietary groups showed no significant difference except in 0.1% STC supplemented group which could be due to the combined effect of sea tangle and charcoal.
The higher concentrations of HDL and lower concentration of LDL in the STC diet groups could be due to the combined effect of STC on duck meat, as sea tangle possesses several biological activities such as antioxidant activity, anti-mutagenic activity (Park et al., 2009). Xu et al. (2013) and Suetsuna (2001) reported antioxidant and antimutagenic effects of seaweed reduced the LDL values and increased the HDL values through strengthening enzyme activity. Dietary supplementation of STC did not affect the TBARS value of fresh duck meat, which corroborates the observations of Lee (2004) in rats. The reduced TBARS concentration of duck meat preserved for 1 week upon SCT supplementation may be due to the antioxidant effect of sea tangle.
The higher concentrations of polyunsaturated fatty acids in the STC dietary group were due to the presence of phospholipids and glycolipids. When there is a reduction of environmental temperature, seaweed can accumulate PUFAs, which are consistent with the present study and also support the fact that species living in cold regions contain more PUFAs than those living at higher temperatures (Holdt and Kraan, 2011). The elevated concentration of omega-3 fatty acids in the STC dietary group also supports the findings of Pulz and Gross (2004) and Plaza et al. (2010), who found that seaweed contains substantial amounts of omega-3 fatty acids, which are substances of particular interest in animal feeding due to their anti-microbial and antioxidant properties as well as their biofortification ability of animal products (Kouba and Mourot, 2011). The omega-3 fatty acid concentration in antibiotic treatment was reduced which may be due to the lower activity of the glutathione peroxidase and catalase enzyme activities in antibiotic dietary group. Sukoyan et al. (2005) reported decreased glutathione peroxidase enzyme activity of the glutathione-associated antioxidant system, after treatment with antibiotics. Addition of PUFAs to animal feed resulted in biofortification of animal products as they are essential for human (Gladine et al., 2007; Kouba and Mourot, 2011). Studies have shown that feeding of animals with omega-3 fatty acids increases the content of these substances in milk and meat (Kouba, 2011).
IMPLICATIONS
During the last few decades, antibiotic was extensively used as a growth promoter in the poultry sector and their residual effect is important in terms of public health concerns. The most challenging objective of this study was to mitigate the use of antibiotics in duck production by utilizing unconventional feed resources. The results of this study revealed that, supplementation of STC had no adverse effect on the overall growth performance of growing ducks which was more or less similar to antibiotics. In addition, STC feeding strategy had no significant effect on the chemical composition of duck breast meat except fat content which was increased in 1.0% STC dietary group. An increased HDL concentration was found in 1.0% STC supplemented groups, whereas LDL concentration was low in 0.1% and 1.0% STC groups. The concentration of omega-3 fatty acids was elevated whereas a reduced ratio of n-6/n-3 PUFA ratio was observed upon STC supplementation which was pronounced in 1.0% STC dietary groups. Therefore, it can be concluded that the dietary supplementation of 1.0% STC can be used as a potential alternative to antibiotics in duck production.
ACKNOWLEDGEMENT
The study was supported by the NURI Agency, Republic of Korea.