Supplementing Vitamin E to the Ration of Beef Cattle Increased the Utilization Efficiency of Dietary Nitrogen
Article information
Abstract
The objectives of the trial were to investigate the effects of supplementing vitamin E (VE) on nutrient digestion, nitrogen (N) retention and plasma parameters of beef cattle in feedlot. Four growing Simmental bulls, fed with a total mixed ration composed of corn silage and concentrate mixture as basal ration, were used as the experimental animals. Four levels of VE product, i.e. 0, 150, 300, 600 mg/head/d (equivalent to 0, 75, 150, 300 IU VE/head/d), were supplemented to the basal ration (VE content 38 IU/kg dry matter) in a 4×4 Latin square design as experimental treatments I, II, III and IV, respectively. Each experimental period lasted 15 days, of which the first 12 days were for pretreatment and the last 3 days for sampling. The results showed that supplementing VE did not affect the nutrient digestibility (p>0.05) whereas decreased the urinary N excretion (p<0.01), increased the N retention (p<0.05) and tended to increase the microbial N supply estimated based on the total urinary purine derivatives (p = 0.057). Supplementing VE increased the plasma concentrations of VE, glucose and triglycerol (TG) (p<0.05) and tended to increase the plasma concentration of total protein (p = 0.096) whereas did not affect the plasma antioxidant indices and other parameters (p>0.05). It was concluded that supplementing VE up to 300 IU/head/d did not affect the nutrient digestibility whereas supplementing VE at 150 or 300 IU/head/d increased the N retention and the plasma concentrations of VE and TG (p<0.05) of beef cattle.
INTRODUCTION
Vitamin E (VE) plays important roles in animal growth, development and reproduction (Liu et al., 1995; McDowell et al., 1996; Rooke et al., 2004). In the quality of beef and mutton, it was reported that supplementing VE enhanced the anti-oxidation and palatability characteristics. When 500 IU VE/head/d (equivalent to 76 IU/kg dry matter [DM]) was added to the ration of cross bred steers fed with wet distiller’s grain-based diets it reduced the lipid oxidation and drip loss (Bloomberg et al., 2011). When 45 mg VE/head/d was supplemented to the barley-based ration during a 75-day fattening period of Awassi male lambs it tended to maintain meat redness of mutton (Macit et al., 2003). In the performance of beef cattle, it was observed that adding up to 500 IU VE/head/d to the finishing ration of cross breed steers (equivalent to 76 IU/kg DM) resulted in a linear increase in carcass-adjusted body weight in average daily gain (Burken et al., 2012) and heifers receiving 570 IU VE/head/d tended to have a higher dressing percentage than receiving 285 IU VE/head/d (Rivera et al., 2002). In in vitro rumen fermentation, it was reported that supplementing VE increased the total volatile fatty acid production and improved the growth of rumen microorganisms (Hino et al., 1993; Naziroğlu et al., 2002; Hou et al., 2013). It could be hypothesized that the positive influence of VE on growth and fattening of beef cattle could result from the effects of VE on nutrient digestibility and N metabolism.
In China, the typical rations for beef cattle usually contain more than 50% of roughages including dried corn stover, corn silage, wheat straw, rice straw and other agricultural byproducts which contain a low content of VE. In beef production, VE is not supplemented to the rations of beef cattle since it is expensive. It is unclear if supplementing VE to the ration of beef cattle is beneficial to the dietary nutrient digestion and utilization. The objectives of the trial were to study the effects of supplementing VE on nutrient digestion, nitrogen (N) retention and plasma parameters of beef cattle.
MATERIALS AND METHODS
Animals and feeding
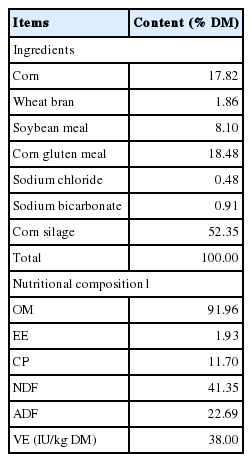
Four Simmental bulls, aged at 12 months, with average live weight of 320±15 kg and fitted with permanent rumen fistulas made of polyethylene, were used as the experimental animals. The cattle were housed in separated pens with rubber mattress in a feedlot and fed with a total mixed ration (TMR) (Table 1) prepared everyday and mixed by hand. The TMR included 8.0 kg/d corn silage and 2.0 kg/d concentrate mixture (DM intake 3.73 kg/d), supplying major nutrients to the cattle at the levels of about 1.1 times of maintenance requirements (Feng, 2000). The corn silage was made from a conventional dent hybrid corn species harvested in October at early milk-line period, chopped to 1–2 cm in length and then filled in a bunker silo (length×width×height: 70 m×25 m×3 m). The silage was used after ensiled for 45 days. The corn silage contained DM 24.43%, organic matter (OM) 89.80% DM; ether extract (EE) 1.97% DM, neutral detergent fibre (NDF) 59.18% DM, acid detergent fibre (ADF) 36.73% DM and crude protein (CP) 7.14% DM. The TMR was divided into two equal meals and fed at 7:00 and 17:00, respectively. The cattle had free access to clean drinking water.
Experimental design
Four levels of VE product (all-rac-α-tocopheryl acetate, VE purity 50%) from DSM China Ltd, i.e. 0, 150, 300, 600 mg/head/d (equivalent to 0, 75, 150, 300 IU/d or 0, 20.1, 40.2, 80.4 IU/kg DM, 1 mg all-rac-α-tocopheryl acetate = 1 International Unit), were supplemented to the basal ration (VE content 38 IU/kg DM) in a 4×4 Latin square design as experimental treatments I, II, III, and IV, respectively. Since the VE content of the TMR was 38 IU/kg DM, the total VE supply to the cattle in four treatments was 142, 217, 292, and 442 IU/d, respectively. Each experimental period was 15 days, of which the first 12 days were for pretreatment and the last 3 days for sampling.
Sampling
During the last 3 days of each experimental period, the faeces and the urine were completely collected at 10:00 daily using the method similar to Dermauw et al. (2013). The faeces from each animal were collected using a plastic bucket and 3% of the faeces were sampled. The urine from each animal was collected using a urine collecting apparatus consisted of a rubber funnel connected to a polyvinyl chloride pipe and a plastic barrel. The rubber funnel was harnessed in the position to the penis by flexible hose straps. An aliquot of 1% of the urine was sampled. On the last sampling day at 11:00, 10 mL of blood sample was taken from the jugular vein of each animal using vacutainers containing Na2-EDTA (Greiner Bio-One GmbH, Frickenhausen, Germany) and were centrifuged at 2,200×g for 15 min to obtain plasma. All the samples were kept at 20°C for later analysis.
Determinations and chemical analysis
The DM, ash, CP, and EE of the samples were determined according to AOAC (2007). The NDF and ADF of the samples were analyzed using the methods of Van Soest et al. (1991). The OM was calculated by DM minus ash. The VE content of TMR and the VE product was analyzed using high performance liquid chromatography (Agilent 1200 HPLC, Agilent Technologies Inc., Santa Clara, CA, USA) according to China National Standards GB/T 17812-2008 and GB/T 7293-2006, respectively. The urinary purine derivatives (PD) including allantoin and uric acid were analysed using the method of Chen and Gomes (1992) on a spectrophotometer (UV-9100, Beijing Beifen Ruili Instrument Co. Ltd, Beijing, China). The VE concentration in plasma was determined using the VE kit (Nanjing Jiancheng Bioengeneering Institute, Nanjing, China). The plasma malondialdehyde (MDA), glutathione peroxidase (GSH-PX), superoxide dismutase (SOD), total antioxidation capacity (T-AOC) and catalase (CAT) were analyzed using the kits HY-50116, HY-60005, HY-60001, and HY-50121, respectively (Beijing Sino-UK Institute of Biological Technology, Beijing, China). The plasma total protein (TP), plasma urea nitrogen, glucose (GLU) and triglycerol (TG) were analyzed using the biuret method, the enzyme coupling ratio method, the GLU oxidative method and the glycerophosphate oxidase-peroxisome method using kits, respectively (Beijing Sino-UK Institute of Biological Technology, China). The plasma insulin-like growth factor-1 (IGF-1), growth hormone (GH) and insulin (INS) were analyzed using the radioimmunoassay kits HY-082, HY-10035, and HY-10069, respectively (Beijing Sino-UK Institute of Biological Technology, China). The plasma samples were analyzed twice as duplicates for every plasma parameter to minimize the errors from analytical procedures.
Calculations and statistical analysis
The absorption of microbial purines and the rumen microbial N supply were estimated according to Chen and Gomes (1992):
where X refers to the absorption of microbial purines, mmol/d; Y, the excretion of PD in urine, mmol/d; W0.75, the metabolic body weight of cattle, kg; 0.385, the endogenous contribution to PD excretion, mmol/kg W0.75; 0.85, the recovery rate of absorbed purines in urine.
where X refers to the absorption of microbial purines, mmol/d; 70, the N content of purine, mg N/mmol; 0.83, the digestibility of microbial purines; 0.116, the ratio of the purine-N in the total N of mixed rumen microbes.
The data were analyzed using the general linear model procedure of SAS 9.1 (SAS Institute Inc, 2003). The model used for the analysis was: Y = μ+VEi+Periodj+Cattlek+ɛ, where Y = observation, μ = general mean, VE = effect of VE (i = 1 to 4), Period = effect of period (j = 1 to 4), and Cattle = effect of cattle (k = 1 to 4), ɛ = residual error. Differences between treatments were determined by Student-Newman-Keuls multiple-range test and considered to be significant at p<0.05, extremely significant at p<0.01 and tended to be significant at 0.05<p<0.10.
RESULTS
The results in Table 2 show that supplementing VE up to 300 IU/head/d did not affect the digestibility of DM, OM, EE, NDF, ADF, and CP of ration (p>0.05) while it decreased the urinary N excretion (p<0.01). Supplementing VE at 150 or 300 IU/head/d increased the N retention (p<0.05) and tended to increase the urinary PD excretion (p = 0.082) and consequently the estimated rumen microbial N synthesis (p = 0.057) of the cattle.
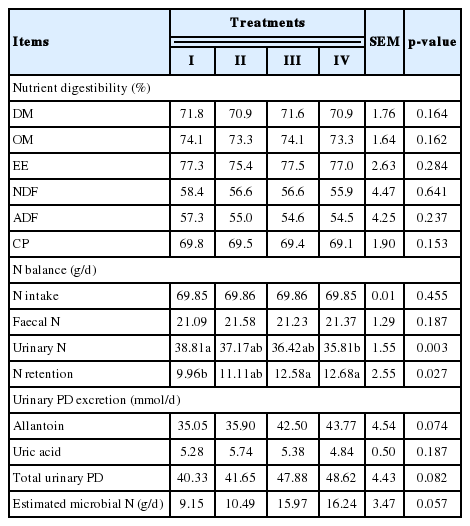
Effects of supplementing vitamin E on nutrient digestibility, N balance, urinary purine derivatives excretion and estimated microbial N supply in beef cattle
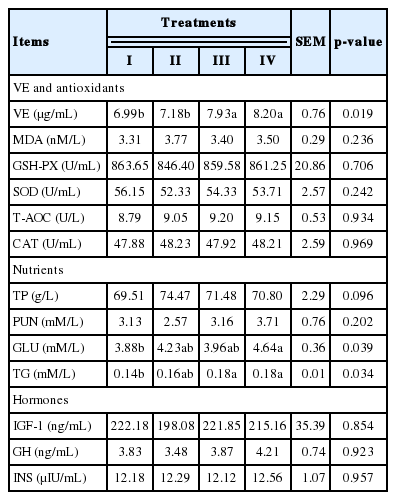
The results in Table 3 showed that supplementing VE at 150 or 300 IU/head/d increased the plasma concentration of VE and TG (p<0.05) and supplementing VE at 300 IU/head/d increased the plasma concentration of GLU (p<0.05). Supplementing VE up to 300 IU/head/d tended to increase the plasma concentration of TP (p = 0.096) whereas did not affect the plasma antioxidants and hormones including IGF-1, GH and INS of the cattle (p>0.05).
DISCUSSION
Vitamin E requirement of beef cattle
McDowell et al. (1996) suggested that the optimal VE requirement of feedlot cattle (growing and finishing) was 200 to 500 IU/head/d. In the present trial, the VE supply from the basal ration for the cattle was 142 IU/head/d (38 IU/kg DM×3.73 DM intake) which was lower than the amount suggested by McDowell et al. (1996). Therefore, supplementing VE at 75, 150, and 300 IU/head/d in treatments II, III, and IV, respectively was presumed to have met the VE requirement of the cattle in the present trial (Table 2).
Nutrient digestibility
Khodamoradi et al. (2013) reported that supplementing 145 mg VE/head/d (equivalent to 7.5 mg/kg DM) to a TMR of lactating Holstein cows had no effects on the digestibility of DM, OM, CP, and EE and on the milk yield and the contents of milk fat, protein and lactose and the N utilization efficiency. The reason could be that the VE content of the basal ration met the VE requirement of the cows or the supplementation level of 145 mg VE/head/d was not high enough to influence the nutrient digestibility and the performance of the cows. The results in the present trial showed that supplementing VE up to 300 IU VE/head/d (equivalent to 80.4 IU/kg DM) did not affect the nutrient digestibility of beef cattle. Chikunya et al. (2004), however, reported that supplementing 500 IU VE (α-tocopheryl acetate)/kg DM to the ration of sheep with 50 g fatty acids/kg DM using three lipid sources increased the whole tract digestibility of cellulose. The differences found between different trials could be attributed to the VE requirements of different animal species, the VE contents in the basal rations and the VE doses.
N utilization
The rumen environment of adult ruminants is suitable for the colonization and growth of rumen anaerobic microorganisms. However, a limited amount of oxygen may go into the rumen from ingestion, rumination and drinking water. Some oxygen may also diffuse from the blood into the rumen. Stewart and Bryant (1988) reported that the concentration of oxygen present in rumen fluid could be as high as 3 mmol/L. Therefore, supplementing VE would be beneficial to the rumen environment for the colonization and growth of rumen microorganisms.
The results in the present trial showed that supplementing VE at 300 IU/head/d decreased the urinary N excretion and supplementing VE at 150 or 300 IU/head/d increased the N retention of beef cattle. The reasons for the results could be that VE was an antioxidant and maintained the integrity of cell membranes from oxidation (Burton et al., 1990) resulted from oxygen taken in from rumination, drinking water and diffusion through the rumen epithelium from the blood and therefore improved the growth of rumen protozoa and bacteria (Hino et al., 1993; Naziroğlu et al., 2002). The results were in agreement with the increased urinary PD excretion, the estimated microbial N supply and the increasing tendency of the plasma concentration of TP.
Urinary purine derivatives and estimated microbial N supply
The urinary PD excretion was directly associated with the intestinal absorption of purine and could be used for the estimation of rumen microbial N supply to ruminants (Chen and Gomes, 1992). The results in the present trial indicated that supplementing VE to the ration of beef cattle tended to increase the urinary PD excretion and consequently the estimated rumen microbial N supply. The results were in agreement with Chikunya et al. (2004) who reported that supplementing 500 IU VE (α-tocopheryl acetate)/kg DM to the rations of sheep increased the estimated microbial N yield compared to 100 IU/kg DM. The results implied that the rumen microorganisms could possibly require VE which was an antioxidant and VE could protect the integrity of microbial membranes from oxidation (Burton et al., 1990).
Plasma vitamin E and antioxidants
Weiss and Wyatt (2003) reported that supplementing up to 5,500 IU VE/head/d (equivalent to 250 IU VE/kg DM) to the ration of mid-lactation Holstein dairy cows increased the plasma concentration of VE (α-tocopherol). Lindqvist et al. (2011) reported that supplementing 2,400 IU α-tocopheryl acetate/head/d (equivalent to total 150 to 169 IU/kg DM) to the ration of Holstein dairy cows during the transition period tended to increase the plasma concentration of VE (α-tocopherol). Chikunya et al. (2004) reported that supplementing 500 IU VE/kg DM to the ration of sheep with 50 g fatty acids/kg DM increased the plasma concentration of VE (α-tocopherol). The results in the present trial indicated that supplementing VE at 150 or 300 IU/head/d increased the plasma VE concentration of beef cattle. The reason for the results could be that VE was not destroyed by ruminal microbes (Leedle et al., 1993). Therefore, supplementing VE increased the plasma concentration of VE. Liu et al. (2008) reported that supplementing 5,000 or 10,000 IU DL-α-tocopheryl acetate/head/d (equivalent to 262 or 563 IU/kg DM) to the ration of Holstein dairy cows did not increase the plasma concentration of GSH-PX, but increased GSH-PX when combined with selenium. In the present trial, supplementing VE up to 300 IU/head/d did not affect the plasma concentration of GSH-PX and other antioxidants including SOD, MDA, T-AOC, and CAT. The results of the present trial were in agreement with Liu et al. (2008) and Bourne et al. (2007). Mahmoud et al. (2013), however, reported that injecting 5 mg sodium selenite and 450 mg VE injections (Viteselen 15, Adwia Company, Tanta, Egypt) two times per week for 1 month increased the serum concentration of GSH-PX of Ossimi rams (VE content in basal ration 15 mg/kg on fed basis). Similarly, Liu et al. (2008) reported that supplementing 5,000 or 10,000 IU DL-α-tocopheryl acetate/head/d (equivalent to 262 or 563 IU/kg DM) to the ration of Holstein dairy cows combined with selenium increased the plasma concentration of GSH-PX. The results indicated that the antioxidants such as the plasma concentration of GSH-PX could not be increased only if the plasma concentration of VE reached a certain level and the combination of VE and selenium was effective to increase the antioxidants of animals.
CONCLUSION
Under present feeding regimes, supplementing VE up to 300 IU/head/d did not affect the nutrient digestibility when the VE content of the basal ration was 38 IU/kg DM. Supplementing VE at 150 or 300 IU/head/d improved the utilization efficiency of dietary N and increased the plasma concentrations of VE, GLU, and TG of beef cattle.
ACKNOWLEDGMENTS
The authors thank DSM China Ltd. for financial support for the trial and also thank Beijing Dairy Industry Innovation Team for assistance in research.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.