Effects of Xylanase Supplementation on Growth Performance, Nutrient Digestibility and Non-starch Polysaccharide Degradation in Different Sections of the Gastrointestinal Tract of Broilers Fed Wheat-based Diets
Article information
Abstract
This experiment was performed to investigate the effects of exogenous xylanase supplementation on performance, nutrient digestibility and the degradation of non-starch polysaccharides (NSP) in different sections of the gastrointestinal tract (GIT) of broilers fed wheat-based diets. A total of 120 7-day-old Arbor Acres broiler chicks were randomly allotted to two wheat-based experimental diets supplemented with 0 or 1.0 g/kg xylanase. Each treatment was composed of 6 replicates with 10 birds each. Diets were given to the birds from 7 to 21 days of age. The results showed that xylanase supplementation did not affect feed intake, but increased body weight gain of broiler at 21 day of age by 5.8% (p<0.05) and improved feed-to-gain ratio by 5.0% (p<0.05). Xylanase significantly increased (p<0.05) ileal digestibilities of crude protein (CP) by 3.5%, starch by 9.3%, soluble NSP by 43.9% and insoluble NSP by 42.2% relative to the control group, respectively. Also, compared with the control treatment, xylanase addition increased (p<0.05) total tract digestibilities of dry matter by 5.7%, CP by 4.1%, starch by 6.3%, soluble NSP by 50.8%, and had a tendency to increase (p = 0.093) insoluble NSP by 19.9%, respectively. The addition of xylanase increased the concentrations of arabinose and xylose in the digesta of gizzard, duodenum, jejunum, and ileum (p<0.05), and the order of their concentration was ileum>jejunum>duodenum>>gizzard> caecum. The supplementation of xylanse increased ileal isomaltriose concentration (p<0.05), but did not affect the concentrations of isomaltose, panose and 1-kestose in the digesta of all GIT sections. These results suggest that supplementation of xylanase to wheat-based diets cuts the arabinoxylan backbone into small fragments (mainly arabinose and xylose) in the ileum, jejunum and duodenum, and enhances digestibilites of nutrients by decreasing digesta viscosity. The release of arabinose and xylose in the small intestine may also be the important contributors to the growth-promoting effect of xylanase in broilers fed wheat-based diets.
INTRODUCTION
In China, wheat has become an important source of energy in poultry diets because of a shortage of corn supply and the increase of corn price. However, the utilization efficiency of wheat is lower than that of corn because it contains more anti-nutritive factors, especially non-starch polysaccharides (NSP). Arabinoxylans are the major NSP fractions in wheat, which increase digesta viscosity, reduce the digestibility of nutrients and decrease the feed efficiency and growth performance when fed to poultry, especially in broiler chickens (Choct and Annison, 1992a; Friesen et al., 1992). Moreover, dietary NSP can also accelerate small intestinal fermentation by modulating the intestinal microflora (Choct et al., 1996; Nian et al., 2011), which might be detrimental to nutrient digestion and absorption for chickens (Choct et al., 1999).
Nowadays, there is no doubt about the usefulness of exogenous microbial NSP-hydrolysing enzymes in wheat-based poultry diets. Xylanase has been widely added to commercial wheat-based compound feeds for broilers to overcome the anti-nutritional effects of NSP. Some previous studies have demonstrated that addition of xylanases in wheat-based diets can reduce the intestinal viscosity by partially hydrolyzing NSP of wheat, resulting in improvements in nutrient digestilities and growth performance of broilers (Choct et al., 2004; Gao et al., 2008; Vandeplas et al., 2010). Moreover, some other studies showed that xylanase supplementation of a wheat-based diet improved chicken immunity (Gao et al., 2007), reduced detrimental effect after Salmonella Typhimurium infection (Vandeplas et al., 2009), or alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens (Liu et al., 2012).
It is generally conceded that the beneficial effects of feed xylanase are primarily the reduction in the viscosity. The ability of xylanase to prevent the formation of viscous digesta appears to be the partial hydrolysis of NSP in the upper digestive tract, leading to a decrease of digesta viscosity in the small intestine (Bedford and Classen, 1992), and elimination of the nutrient encapsulating effect of the cell wall polysaccharides, and a release of the enclosed nutrients (Choct and Annison, 1992a; Meng et al., 2005). Gao et al. (2008) speculated that the growth-promoting effect of xlyanase may be also related to some oligosaccharides in the digesta of the gut produced by exogenous or endogenous enzymes. However, much less information is available on the effects of dietary xylanase supplementation on the NSP degradation and the release of some simple sugars and oligosaccharides in different sections of the gastrointestinal tract (GIT) of broilers fed wheat-based diets. Therefore, the objective of this study was to evaluate the effects of xylanase supplementation on growth performance, nutrient digestibilities, NSP degradation and the release of some simple sugars and oligosaccharides in different sections of the GIT of broilers fed wheat-based diets.
MATERIALS AND METHODS
Enzyme preparation
The enzyme supplement was provided by Jiahe Feed Biotechnology Co., Ltd, Hangzhou, China. The xylanase was derived from a genetically modified isolate of Aspergillus niger, consisting of 3,200 U/g enzyme activity determined using the 3,5-dinitrosalicylic acid reducing sugar method with oat xylan as substrate (Bailey, 1988). One unit of xylanase activity is defined as the amount of enzyme which, at 40°C, pH 5.3, liberates 1 mmole of reducing sugars (expressed as equivalents per min) from xylan.
Birds, diets and experimental design
A total of 200 1-day-old broiler chickens (Arbor Acres strain, AA) were purchased from a commercial hatchery (Hewei agricultural development Co., Ltd, Anhui, China). All chickens were fed a commercial broiler starter during the first 7 days. At the age of 7 day, 120 birds were selected by weight and randomly allotted to 2 wheat-based experimental diets supplemented with 0 or 1.0 g/kg xylanase. The ingredient composition and nutrient levels of the basal diet are shown in Table 1. Each treatment group was composed of 6 replicates with 10 birds each (ten birds per cage). Diets were given to the birds from 7 to 21 days of age. The birds were kept in wired 3-level battery cages (75 cm×45 cm) from 7 to 21 days and housed in an environmentally controlled room. The room was lit continuously during the whole experimental period and room temperature was controlled at 35°C from 0 to 3 day and then gradually reduced by 2°C to 3°C per week to a final temperature of 28°C. Diets were fed in mash form and birds were allowed ad libitum access to feed and water. On day 21, body weight (BW) was recorded for each replicate, and feed consumption was also recorded. The body weight gain (BWG), feed intake, and feed conversion ratio (FCR; feed:gain, g:g) were calculated. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University.
Samples collection
On the last 3 days of the experiment (on day 19 to 21), mixed excreta samples from each cage were total collected. The excreta samples were then dried at 60°C and finally ground for chemical analyses.
At the age of 21 days, 12 birds (2 birds per replicate) were randomly selected and killed by cervical dislocation. The body cavity was opened and the GIT was removed and ligated into eight segments: crop, proventriculus, gizzard, duodenum, jejunum (from duodenum to the Meckel’s diverticulum), ileum (from the Meckel’s diverticulum to 1 cm above the ileo-caecal junction), caecum and colon. The GI tract sections were squeezed lightly by hand and the digesta samples were collected. The digesta samples from the 2 birds within a cage were pooled in sealed bags and stored at −20°C for the analyses of the monosaccharides and oligosaccharides. A subsample of the ileal digesta was dried at 60°C and ground for chemical analyses.
Chemical analysis and calculations
Diet, digesta and excreta samples were analyzed for the contents of dry matter (DM), crude protein (CP), fat, and ash using the AOAC procedures (2000). Starch content was measured based on the amyloglucosidase-α-amylase method as described by McCleary et al. (1997). The soluble and insoluble NSP contents of diet, ileal digesta and excreta were determined according to the methods described by Englyst et al. (1994) and Quigley et al. (1999). Acid-insoluble ash (AIA) was determined as an indigestible marker following the method described by Vogtmann et al. (1975) and Choct and Annison (1992b). The following equation was used for calculation of apparent ileal or total tract digestibility of nutrients:
Free sugar measurement
The concentrations of glucose, galactose, arabinose, xylose, mannose and ribose in the digesta samples were evaluated by using gas chromatography (GC). This was modified from the method of Quigley et al. (1999) as follows.
The standards of glucose (Sigma G7021), arabinose (Sigma A3131), xylose (Sigma X1500), galactose (Fluka 48260), mannose (Fluka 63580) and ribose (Sigma R7500) were purchased from Sigma–Aldrich Co., St. Louis, MO, USA. All of them were dried to constant mass, and were (a weight of 200 mg of each sugar standard) diluted to 200 mL with 50% saturated benzoic acid to obtain standard stock solutions at 1 mg/mL and then stored at 4°C for preparing working sugar standards. Milli-Q water (Millipore, Milford, MA, USA) was used throughout. All other chemicals and solvents were of analytical reagent or gas chromatographic grade.
The freeze-dried digesta sample (0.5 g) was dissolved in 12.5 mL 80% (v:v) ethanol. The tubes were then placed at 10 second intervals into an oscillating rack in a water bath maintained at 40°C for 1 h. After centrifugation at 1,800×g for 10 min, 2 mL of supernatant fraction was transferred to a new tube for GC analysis.
The monosaccharide contents in the reaction mixture were analyzed on a Shimadzu GC-14B gas chromatograph (Shimadzu Corp., Kyoto, Japan) equipped with a DB-225 capillary column (30 m×0.25 mm id; J&W Scientific Inc., Folsom, CA, USA) and a flame-ionization detector (FID). The column temperature was kept at 195°C for 2 min, raised to 235°C at 15°C/min and maintained at this temperature for 10 min. The temperatures of the injector and detector were set at 300°C. A volume of 1 μL of sample was used for GC analysis.
Oligosaccharide measurement
The standards of isomaltose (Supelco 47268-U), isomaltotriose (DP3, Supelco 47884), panose (D-panose, Sigma P2047) and 1-kestose (Fluka 72555) were purchased from Sigma-Aldrich. The freeze-dried digesta sample (0.5 g) was oximated with 0.5 mL oximating agents (dissolving 5 g of hydroxylamine hydrochloride in 50 mL of pyridine) for 15 min at 60°C until dried, and then silylated with 0.5 mL N-(trimethylsilyl)imidazole for 5 min. The reaction mixture is then neutralized with 0.2 mL ultrapure water and 0.4 mL isooctane. After centrifugation at 1,800×g for 10 min, the upper organic phase was collected for oligosaccharide concentrations using GC analysis modified from Joye and Hoebregs (2000). The GC analyses were carried out on a Finnigan Trace GC 2000 equipped with a FID, using a HP-5 MS capillary column (30 m×0.25 mm id, 0.25 μm). The GC-MS analysis was performed on a Finnigan Trace GC 2000 equipped with a Finnigan DSQ mass spectrometer, using the same column described above. The detailed experimental conditions were as follows: injection volume, 1 μL; injection temperature, 330°C; detector temperature, 330°C; column temperature programmed from 200 to 290°C at 5°C/min, holding for 5 min at 290°C, then increasing to 310°C at 10°C/min and finally holding for 3 min at 230°C. Nitrogen was used as the carrier gas at a flow rate of 1.2 mL/min.
Statistical analyses
Data of growth performance and digestibility were performed by independent-sample t-test with SPSS statistical software (version 11.0 for Windows, SPSS Inc., Chicago, IL, USA). Data of the concentrations of free sugars and oligosaccharides in different sections of GIT were analyzed as a 2×6 factorial arrangement by a general llinear model procedure (SPSS 11.0). The variance model included the main effects of the xylanase supplemental level, sections of GIT and their interaction. The results were presented by mean values and the standard error of the mean (SEM). All statements of significance are considered on a p-value less than 0.05.
RESULTS
Growth performance
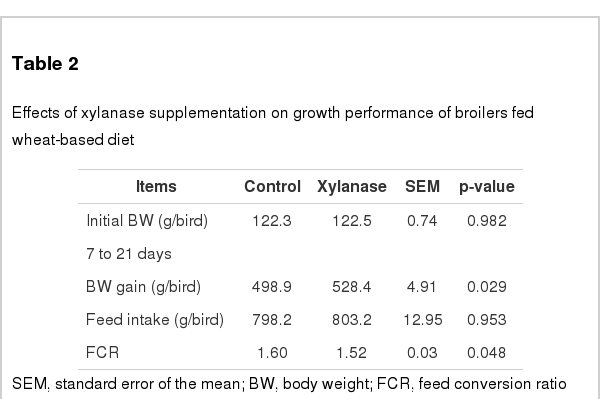
The growth performance of broiler chickens fed diets containing xylanase from 7 to 21 days of age was present in Table 2. Xylanase supplementation significantly increased BWG of broiler at 21 day of age by 5.8% (p<0.05) and improved FCR by 5.3% (p<0.05) compared to those birds fed xylanase-free diets. No significant difference on feed intake was observed between treatments (p>0.05).
Digestibility
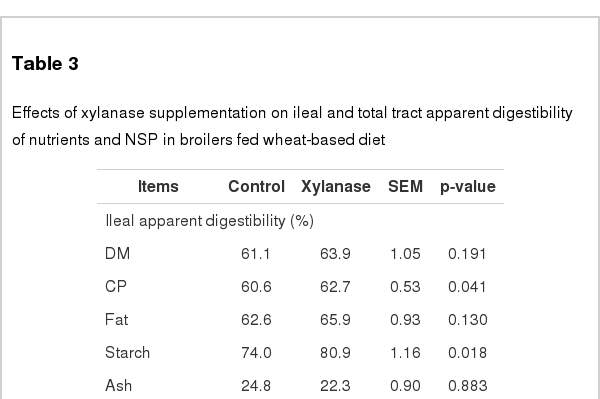
As shown in Table 3, xylanase significantly increased (p<0.05) ileal digestibilities of CP by 3.5%, starch by 9.3%, soluble NSP by 43.9% and insoluble NSP by 42.2% relative to the control group, respectively. Also, compared with the control treatment, xylanase addition increased (p<0.05) total tract digestibilities of DM by 5.7%, CP by 4.1%, starch by 6.3%, soluble NSP by 50.8%, and had a tendency to increase (p = 0.093) insoluble NSP by 19.9%, respectively. The ileal and total tract digestibilities of fat and ash, as well as ileal DM digestibility was not affected by xylanase supplementation (p>0.05).
Concentrations of free sugars and some oligosaccharides in digesta
Xylanase addition increased the concentrations of arabinose and xylose in the digesta of gizzard, duodenum, jejunum and ileum (p<0.05, Table 4), and the order of their concentration was ileum>jejunum>duodenum>>gizzard> caecum. Interactions between xylanase supplemental level and sections of GIT on concentrations of arabinose and xylose were observed (p<0.05). No significant differences in the concentrations of ribose, mannose, galactose and glucose in digesta samples of all the GIT sections was observed between treatments (p>0.05). The concentrations of all these 5 kinds of free sugars were lowest in the caecal digesta.
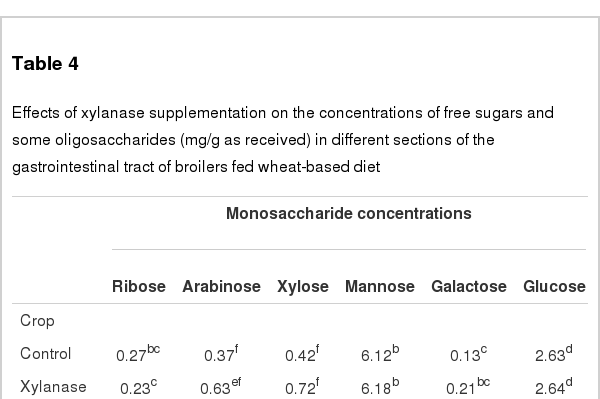
Effects of xylanase supplementation on the concentrations of free sugars and some oligosaccharides (mg/g as received) in different sections of the gastrointestinal tract of broilers fed wheat-based diet
No significant effect was found in the concentrations of isomaltose, panose, and 1-kestose in digesta samples of all the GIT sections between treatments (p>0.05, Table 4). Ileal isomaltriose concentration was higher in birds fed the diet supplemented with xylanase compared to the control diet (p<0.05), and the order of its concentration was jejunum>duodenum>ileum.
DISCUSSION
In accordance with previous reports (Gao et al., 2007; 2008; Esmaeilipour et al., 2011), the data of the present study once more confirmed that the addition of xylanase to wheat-based diets significantly increased the BWG and improved the FCR of broilers at 21 day of age. In this study, xylanase supplementation did not affect the feed intake of birds, indicating that the improvement in feed efficiency observed is likely to be a result of the improved nutrient utilization.
It is well recognized that water-soluble arabinoxylans are major anti-nutritive factors of NSP in wheat-based diets, which increase digesta viscosity, enclose nutrients, modulate gut microflora and, thus, interfere with digestion and absorption of the nutrients (Choct and Annison, 1992a; Choct et al., 1996). The present study demonstrated that addition of xylanase to wheat-based diet has the capacity to increase ileal and total tract digestibilities of CP and starch (Table 3), which could be the direct reason for the improvement of growth performance in broilers. Our results are partially consistent with the findings of Esmaeilipour et al. (2012) who reported that xylanase significantly decreased the viscosity of digesta and improved apparent total tract nutrient digestibilities of DM, CP and energy in broiler chickens fed wheat-based diets from 1 to 24 days of age. In a previous study, Gao et al. (2007) reported that the addition of xylanase to wheat-based diet increased the apparent total tract digestibility of fat. However, no significant effect of xylanase on both ileal and total tract digestibilities of fat were observed in this study, which is in accordance with several previous studies (Bedford, 2000; Esmaeilipour et al., 2012). Therefore, the present findings, along with those results from Bedford (2000) and Esmaeilipour et al. (2012), suggest that xlyanase can reduce nutrient entrapment and increase their digestibilities, mostly starch and protein rather than fat.
The cell walls of cereals contain up to 15% NSP, which are comprised of soluble and insoluble NSP (Diebold et al., 2004). The insoluble fraction of NSP makes up the bulk of the total fibre in diets, and they are traditionally regarded as a nutrient dilution agent and have little or no effect on nutrient utilization in monogastric animals (Choct, 1997; Hetland et al., 2004). However, the soluble fraction of NSP, mainly arabinoxylans in wheat, can not only act as a physical barrier to nutrient digestion and absorption by increasing gut viscosity, but also change digestive tract functions by modifying secretion of endogenous digestive enzymes, water, and electrolytes, as well as elevating fermentation in the small intestine (Choct 1996; Choct et al., 1997). In a recent study, Barekatain et al. (2013) reported that addition of xylanase to diets containing sorghum distillers’ dried grains with soluble (sDDGS) significantly reduced the concentration of insoluble NSP, and increased the concentration of free sugars (arabinose and xylose) in the ileal digesta. They explained that the availability of these free sugars may have provided nutrients to the birds, leading to improved FCR. In the present study, addition of xylanase to wheat-based diet significantly increased the ileal and total tract digestibilities of both soluble and insoluble NSP. Therefore, it can be concluded that exogenous xylanase eliminates the anti-nutritive effect of NSP by partial hydrolysis of both soluble and in soluble NSP, which leads to the reduction of gut digesta viscosity, and the increase of nutrient digestibility. Moreover, the higher total tract digestibilities of both soluble and insoluble NSP than those in the ileal digesta may due mainly to the caecal fermentation.
The beneficial effects of feed xylanase are primarily the reduction in the viscosity and, secondarily, the release of sugars (Malathi and Devegowda, 2001; Choct et al., 2004; Barekatain et al., 2013). The release of monosaccharides caused by exogenous enzymes is due to two reasons: firstly, the breakdown of NSP led to release of their respective monosaccharides, and secondly, the breakdown of NSP released the starch within the endosperm, which was exposed to the endogenous amylase, releasing more glucose (Malathi and Devegowda, 2001). However, in the present study, no significant difference in digesta glucose concentrations of different sections of the GIT between treatments was found. We speculate that this phenomenon may be mainly because of the higher glucose absorption in broilers fed xylanase. Choct et al. (2004) reported that xylanases increased the amounts of free sugars by releasing the arabinose and xylose (the two main sugars of wheat arabinoxylans) both in the jejunal and the ileal digesta of birds fed a wheat-based diet. Xylanase also increases the concentrations of arabinose and xylose in the ileal digesta of birds fed a diet containing sDDGS (Barekatain et al., 2013). This study is the first to report the effect of xylanase on the release of sugars in the different sections of GIT of broilers. Our results showed that the addition of xylanase did not affect the digesta concentrations of ribose, mannose, galactose and glucose in all section of the GIT, but increased the concentrations of arabinose and xylose in the digesta of gizzard, duodenum, jejunum and ileum, and the order of their concentration was ileum>jejunum> duodenum>>gizzard>caecum (Table 4). These findings indicate that i) the monosaccharides released from wheat NSP mainly were arabinose and xylose hydrolyed form arabinoxylans, rather than ribose, mannose and galactose; and ii) the main site for arabinoxylans hydrolysis was the small intestine with the order of ileum>jejunum>duodenum.
A world-wide interest in oligosaccharides is on the increase because of their potential prebiotic function (Patel and Goyal, 2011). Fructo-oligosaccharides, such as 1-kestose, nystose and fructosylnystose, are nondigsetable, but they are selectively utilized by the resident microbes, such as lactic acid bacteria and Bifidobacteria in the gut (Kaplan and Hutkins, 2000; Parracho et al., 2007). Isomalto-oligosaccharides, including isomaltose, panose and isomaltotetraose, have been demonstrated to normalize bowel movement, increase stool bulk, stimulate colon microbial activity (i.e., the growth of Bifidobacteria and Lactobacillus), and modulate the immune function (Chen et al., 2001; Hirayama, 2002; Chung and Day, 2004). In the present study, no significant difference on the concentrations of isomaltose, panose and 1-kestose was observed between treatments, except for a higher ileal isomaltriose concentration in birds fed with xylanase, indicating that the growth-promoting effect of xylanase is not due to increased concentrations of these potentially prebiotic oligosaccharides.
CONCLUSIONS
The supplementation of xylanase to wheat-based diets cuts the arabinoxylan backbone into small fragments (mainly arabinose and xylose) in the ileum, jejunum and duodenum, and enhances digestibilites of nutrients by decreasing digesta viscosity. Although dietary xylanase did not affect the concentrations of some potentially prebiotic oligosaccharides, including isomaltose, panose and 1-kestose, in the GIT, the release of arabinose and xylose in the small intestine may also be the important contributors to the growth-promoting effect of xylanase in broilers fed wheat-based diets.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (30300251) and Three Agricultural Projects of Jiangsu province of China (SX(2011)146).