Changes in Microbial Diversity, Methanogenesis and Fermentation Characteristics in the Rumen in Response to Medicinal Plant Extracts
Article information
Abstract
This study evaluated the in vitro effect of medicinal plant extracts on ruminal methanogenesis, four different groups of methanogens and ruminal fermentation characteristics. A fistulated Holstein cow was used as a donor of rumen fluid. Licorice and mugwort extracts (Glycyrrhiza uralensis and Artemisia capillaris, 0.5% and 1% of total substrate DM, respectively), previously used as folk remedies, were added to an in vitro fermentation incubated with buffered-rumen fluid. Total gas production in Glycyrrhiza uralensis extract treatment was not significantly different between treatments (p<0.05) while total gas production in the Artemisia capillaris extract treatment was lower than that of the control. Artemisia capillaris extract and Glycyrrhiza uralensis extract reduced CH4 emission by 14% (p<0.05) and 8% (p<0.05), respectively. Ciliate-associated methanogens population decreased by 18% in the medicinal plant extracts treatments. Medicinal plant extracts also affected the order Methanobacteriales community. Methanobacteriales diversity decreased by 35% in the Glycyrrhiza uralensis extract treatment and 30% in the Artemisia capillaris extract treatment. The order Methanomicrobiales population decreased by 50% in the 0.5% of Glycyrrhiza uralensis extract treatment. These findings demonstrate that medicinal plant extracts have the potential to inhibit in vitro ruminal methanogenesis.
INTRODUCTION
The attention of methane emission has now shifted towards its contribution to global warming. This growing concern about global warming has focused attention on ways to inhibit ruminal methanogenesis. Methane is generated by ruminal microbes (i.e. methanogenic archaea) that utilize H2 to reduce CO2, and this is the primary electron sink in the rumen. Enteric fermentation by ruminants is one of major contributors to biogenic methane formation, and it leads to an unproductive use of dietary energy resulting in the substantial loss of up to 12% of the dietary energy intake (Gibbs et al., 1989; Crutzen, 1995; Johnson and Johnson, 1995). Manipulating the ruminal ecosystem to enhance digestibility of fibrous feeds, and reduce methane emission by ruminants to improve animal performance are most important goals for animal microbiologists and nutritionists. Feed additives such as organic acids, ionophores, halogen compounds and other antibiotics were used to modify ruminal fermentation, affect ruminal methanogenesis and improve animal performance (Chalupa, 1988; Martin et al., 1999; McGuffey et al., 2001; Russell and Houlihan, 2003). However, the use of antibiotics as feed additives in animals is banned in the EU due to the concerns of wide spread resistant bacteria and residues in dairy products (Russell and Houlihan, 2003). Recently, attention has shifted to alternative candidates as a safe means of modifying ruminal fermentation. Plant extracts, including their secondary metabolites, have been used for centuries in traditional medicine, for industrial applications, and as food preservatives. Furthermore, due to their antimicrobial activity, they are being considered as alternatives to antibiotics in ruminant feeds with the potential to reduce ruminal methanogenesis (Davidson and Naidu, 2000; Teferedegne, 2000; Greathead, 2003). The effects of these plant extracts and their constituents on growth performance of Hanwoo calves (Sarker et al., 2010), ruminal methanogenesis (Patra et al., 2006; Patra et al., 2010; Kim et al., 2012), the physicochemical properties and sensory scores of Hanwoo beef (Moon and Jung, 2011), and nutritional composition of Hanwoo beef (Moon and Jung, 2011) were reported. However, there are limited data on effects of these plant extracts or secondary metabolites on ruminal fermentation, their mode of action and optimal dosages to improve the efficiency of nutrient utilization.
The objective of this study was to evaluate the in vitro effects of licorice and mugwort extracts (Glycyrrhiza uralensis and Artemisia capillaris, 0.5% and 1% of total substrate DM, respectively) on ruminal methanogenesis and fermentation characteristics.
MATERIALS AND METHODS
Rumen fluid
A fistulated Holstein cow of 500kg body weight was used as a donor of rumen fluid. Timothy and commercial concentrate (TDN; 73.5%, crude protein; 19%, crude fat; 3%, crude fiber; 12%, crude ash; 10%, Ca; 0.8%, P; 1.2%) in the ratio of 60:40 were fed at 2% of body weight twice a day (09:00 and 18:00). Water and mineral-vitamin block were allowed ad libitum. The rumen fluid was collected from the fistulated Holstein cow before morning feeding. Rumen samples were collected in a bottle, previously kept warm and filled with O2 free-CO2 gas, and carried to the laboratory.
Preparation of medicinal plant extracts
Licorice and mugwort extracts (Glycyrrhiza uralensis and Artemisia capillaris) were prepared in methanol at 100 g/300 mL of solvent. The flasks of methanol were stoppered and incubated at 39°C on a rotary shaker (HBS-201SL, HANBAEK, Korea). They were filtered through filter paper (Whatman No. 1). The filtrates were collected and the solvent was removed in a rotary evaporator, and then stored at 4°C for further use.
In vitro incubation
Rumen liquor was filtered through four layers of cheesecloth before mixing with buffer maintained at 39°C. The 25 mL of rumen fluid-buffer mixture, comprising McDougall buffer (McDougall, 1948) and rumen liquor in the ratio of 4 to 1, was dispensed anaerobically into serum bottles containing 0.3 g of timothy substrate and plant extracts (0.5% and 1% of total substrate DM, respectively). The serum bottles were filled with O2-free N2 gas capped with a rubber stopper and held in a shaking incubator (HBS-201SL, HANBAEK, Korea) at 39°C for 48 h. The in vitro experiment was evaluated in a triplicate run for analysis of gas proflies and VFAs.
Gas production
At the end of incubation, total gas production was measured by the assay of Theodorou et al. (1994). A detachable pressure transducer and a digital readout voltmeter (Laurel Electronics, Inc., CA USA) were used to measure the headspace gas pressure of fermenting cultures. For the total gas production measurement, the transducer was modified in such a way that it could be linked to the inlet of a disposable Luer-lock three-way stopcock (Theodorou, 1994). Gas pressure in the headspace was read from the display unit after insertion of the hypodermic syringe needle through the butyl rubber stopper above the culture medium.
Gas analysis and ruminal fermentation
The headspace gas in the serum bottle was collected for analyzing methane and hydrogen by gas chromatography (GC-2010, Shimadzu, Japan) equipped with column (Shincarbon ST. 50/80, Shimadzu, Japan). The culture was subsampled for the analysis of pH (Mettle-Toledo, CH/MP220), volatile fatty acid (VFA) concentration and gDNA extraction. VFA analysis was performed with a gas chromatography (GC-2010, Shimadzu, Japan) as described by Erwin et al. (1961).
In vitro dry matter disappearance
After centrifugation of the cultures, the residual pellets were filtered with filter paper (Whatman No. 1). Filtered pellets were dried to a constant weight at 60°C. DM disappearance was calculated to the percentage by dividing the differences between filter paper weight and filter paper weight with pellet after dried by the original weight from control culture at zero time incubation equally processed.
DNA extraction
TissueLyser (Retsch; QIAGEN, Valencia, CA), a high-speed reciprocal shaker which retains samples in screw-capped tubes containing silica beads, was used for DNA extraction. Total nucleic acid was extracted from the incubated rumen samples using the modified bead-beating protocol with the QIAamp DNA mini kit (250) (QIAGEN, USA). For samples a 1.0 mL aliquot was taken from the 25 mL incubated culture using a wide bore pipette so as to ensure a homogenous sample containing fluid and digesta. Nucleic acid concentrations were measured by using a NanoDrop Spectrophotometer (ND-1000, USA).
PCR primers
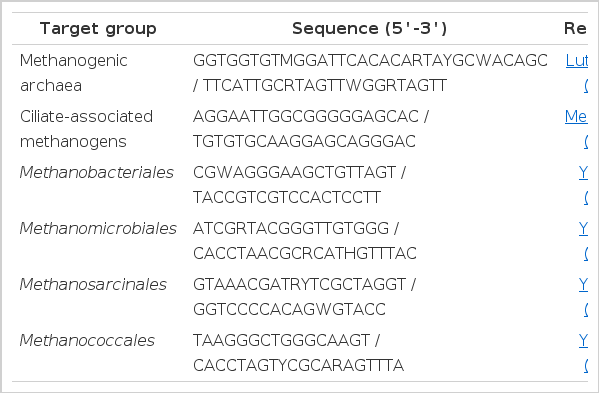
The PCR primer sets used in this study for amplification of ciliate-associated methanogen and four different groups of methanogens (the order Methanobacteriales, the order Methanomicrobiales, the order Methanosarcinales and the order Methanococcales) were the same as referenced by Luton et al. (2002), Medlin et al. (1998) and Yu et al. (2005), respectively as shown in Table 1.
Real-time PCR
Quantitative PCR assays for enumeration of ciliate-associated methanogens and four different groups of methanogens were performed according to the methods described by Denman and McSweeney (2006) and Denman et al. (2007) on a real-time PCR Machine (Rotor-Gene, Crobett life science, Australia) using the iQ SYBR Green Supermix (Bio-Rad Inc. USA). The values of cycle threshold (Ct) after real-time PCR were used to determine fold change (number of fold difference) of different microbial population relative to control without plant extract. Abundance of these microbes was expressed by the equation: relative quantification = 2−ΔCt(Target)−ΔCt(Control), where Ct represents threshold cycle. All quantative (q) PCR reaction mixtures (final volume of 25 μL) contained forward and reverse primers, the iQ SYBR Green Supermix and DNA template ranging from 10 ng to 100 ng. A negative control without the template DNA was used in every qPCR assay for each primer. The PCR conditions including the annealing and the extension temperature were as shown in references (Table 1).
Statistical analysis
Data were analyzed using the general linear model (GLM) procedure of the Statistical Analysis System Institute, Inc. (SAS, 2002). The effects of the plant extracts on total gas, gas profiles, DM, pH, and VFA were compared to the controls and significant differences between treatment means were examined using Duncan’s multiple comparison tests. A p<0.05 was considered to indicate statistical significance. All analyses were carried out using Statistical Analysis Systems (SAS) version 9.1 (2002).
RESULTS AND DISCUSSION
The results of in vitro total gas production including methane and hydrogen (not detected), and dry matter disappearance by medicinal plant extracts are shown in Table 2. Glycyrrhiza uralensis and Artemisia capillaris extracts affected total gas production (mL/g DM) as well as methane emission (mL/g DM). Total gas production in the Glycyrrhiza uralensis extract treatment was not significantly different from that of control, while total gas production in the Artemisia capillaris extract was lower than that of the control (p<0.05, Table 2). Artemisia capillaris extract and the Glycyrrhiza uralensis extract reduced methane emission by 14% (p<0.05) and 8% (p<0.05), respectively, after 48 h of incubation (Table 2). This finding was in agreement with decreased abundance of ciliate-associated methanogen communities and Methanobacteriales populations in this experiment. Kim et al. (2012) also reported that wormwood extract inhibited methane emission by 8% after 24 h of incubation compared with untreated control. Hydrogen produced was not detected. Hydrogen is the critical concern to the ruminal ecosystem. The formation of propionate from succinate would be a hydrogen-sink product alternative to hydrogen for ruminal methanogenesis. Hydrogen is a substrate for methane production in the rumen and regulation of the hydrogen-consuming pathway is the key to control methane emission in the rumen. This study showed that medicinal plant extracts potentially influenced ruminal methanogenesis, resulting in a reduction in methane emission. Although not significant, DM disappearance increased by 7% in the 1% of Glycyrrhiza uralensis and Artemisia capillaris extracts treatments. The ratio of acetate to propionate in the all plant extracts treatments were not significantly different in comparison with that of control (p<0.05, Table 3).
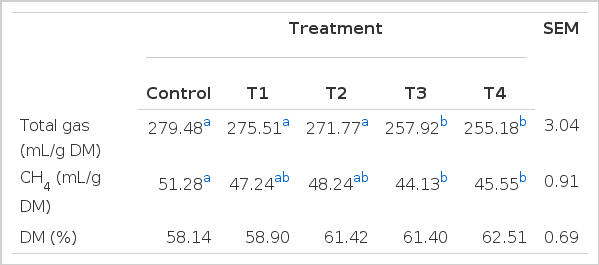
The in-vitro effect of Glycyrrhiza uralensis and Artemisia capillaris extracts on total gas, CH4 emission and ruminal disappearance of dry matter (DM) after 48 h incubation

The in-vitro effect of Glycyrrhiza uralensis and Artemisia capillaris extracts on ruminal fermentation characteristics after 48 h incubation
Plant secondary metabolites including essential oils have been largely used for various purposes in agriculture as food preservatives and feed additives due to their antiseptic and medicinal properties. Glycyrrhiza uralensis and Artemisia capillaris extracts used in this experiment have been used as folk remedies and oriental medicine materials. In the previous study using PCR, the order Methanococcales and the order Methanosarcinales were not detected in any incubated mixtures (Kim et al., 2013). Behlke (2007) reported that both of these two orders (the order Methanococcales and the order Methanosarcinales) were either not in the rumen or they were less than the detection limit. The Ciliate-associated methanogens population was affected by all plant extracts (Figure 1a). Ciliate-associated methanogen populations were decreased by 18% in the Glycyrrhiza uralensis and Artemisia capillaris extracts treatments. Ciliate protozoa have an important role in ruminal methanogenesis by their association with methanogens which adher to their surface. The finding that medicinal plant extracts decreased the ciliated-associated methanogens agrees with evidence that protozoa-free ruminants would result in the reduction of methane emission. Glycyrrhiza uralensis and Artemisia capillaris extract affected the order Methanobacteriales community (Figure 1b). Methanobacteriales community was decreased by 35% in the Glycyrrhiza uralensis extract treatment, and 30% in the Artemisia capillaris extract treatment. The order Methanomicrobiales population was decreased by 50% in the 0.5% of Glycyrrhiza uralensis extract treatment (Figure 1c). According to in vivo experiments, Sarker et al. (2010) reported that licorice among the feed additives investigated may be a suitable source of alternatives to antibiotics for Hanwoo beef calves, especially post-weaning ones. Kim et al. (2006) and Ko et al. (2006) evaluated the effect of replacing rice straw or concentrates with wormwood (Artemisia sp.) on animal performance. Based on the findings, animal performance was better when sheep were fed diets in which 50 or 100 g/kg DM of rice straw was replaced with dry wormwood than those in which 30 g/kg DM of rice straw was substituted. Replacing the concentrate with 30 to 50 g/kg DM wormwood was suitable to optimize ruminal fermentation, increase microbial yield and ether extract digestibility in sheep.
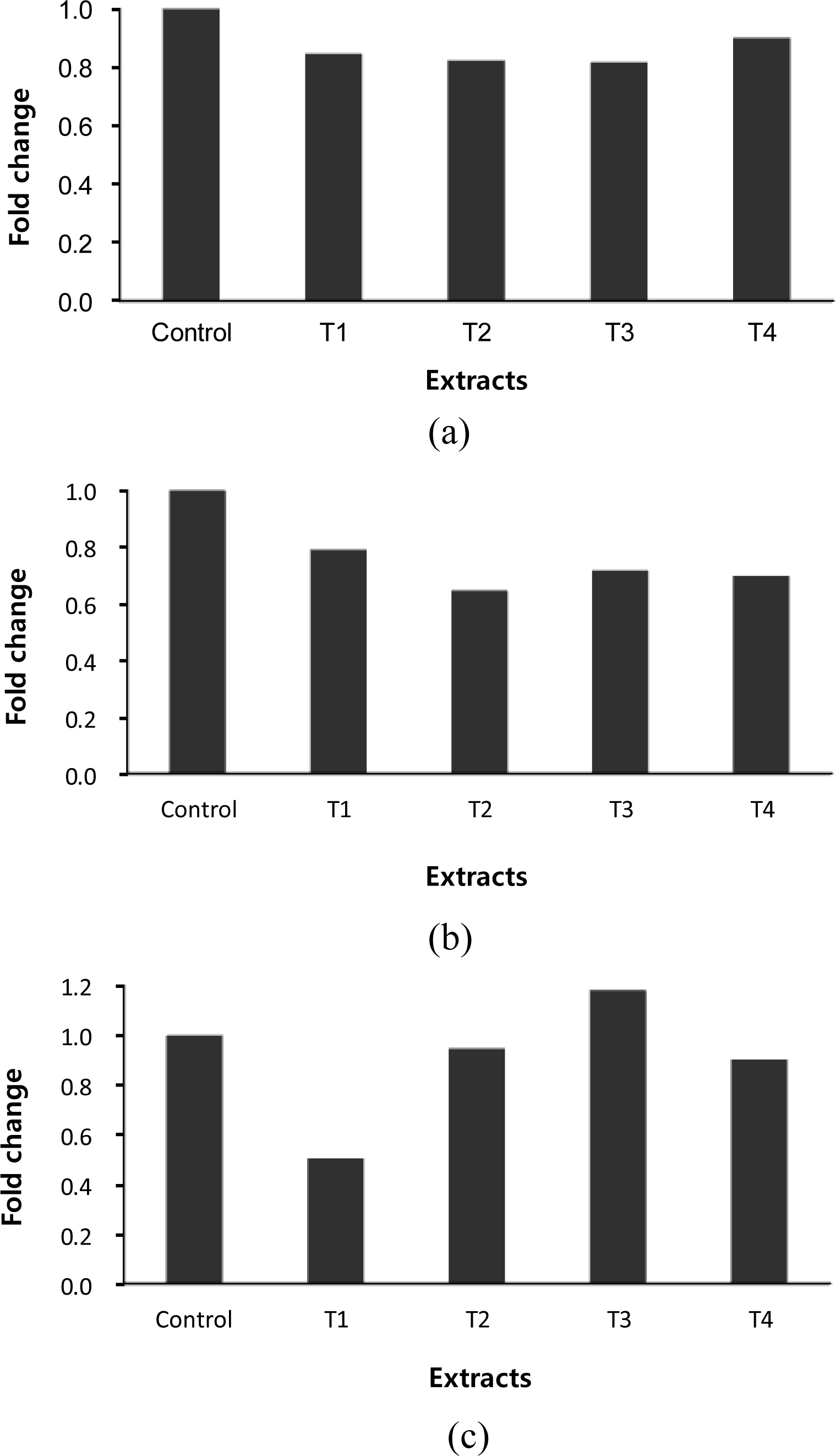
Relative quantification analysis of ciliate-associated methanogens (a), Methanobacteriales (b) and Methanomicrobiales (c) populations in vitro ruminal fermentation with the addition of Glycyrrhiza uralensis and Artemisia capillaris extracts after 48 h incubation (Control = No additive, T1 = 0.5% of Glycyrrhiza uralensis, T2 = 1% of Glycyrrhiza uralensis, T3 = 0.5% of Artemisia capillaris, T4 = 1% of Artemisia capillaris).
In conclusion, Glycyrrhiza uralensis and Artemisia capillaris plant extracts, previously used as folk remedies, inhibited in vitro ruminal methanogens closely related with methane emission although ruminal fermentation characteristics were not shown expected results. It shows that Glycyrrhiza uralensis and Artemisia capillaris plant extracts have the potential to inhibit ruminal methanogenesis, especially considering the optimal dosage of plant extracts.
Acknowledgements
This research was supported by Bio-industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, and Cooperative Research Program for Agriculture Science & Technology Development, Rural Development Administration, Republic of Korea.