Effect of Saccharomyces cerevisiae Fermentation Product on Lactation Performance and Lipopolysaccharide Concentration of Dairy Cows
Article information
Abstract
To evaluate lactation performance and changes in plasma and fecal lipopolysaccharide (LPS) concentrations in response to the supplementation of Saccharomyces cerevisiae fermentation product (SC), two dairy farms were selected. On each farm, 32 cows in early to mid lactation (21 to 140 DIM) were blocked by parity and days in milk (DIM), and randomly assigned to one of the two treatments within block (Control or 56 g SC/cow/d). Effect of SC on lactation performance (daily) and changes in blood and fecal LPS level were examined on d 0 and 28 of supplementation. The results showed that SC supplementation increased lactation performance of dairy cows on both farms. On Farm 1, milk production, 3.5% fat corrected milk (FCM), and yield of milk fat and protein were greater (p<0.01) for cows supplemented with SC. Supplementation of SC increased percentage milk fat (p = 0.029) from 81 to 110 DIM. There was no significant effect (p>0.05) of SC supplementation on percentage of milk protein, dry matter intake and feed efficiency. On Farm 2, cows supplemented with SC had a greater (p<0.05) milk yield, percentage of milk fat and milk protein, yield of milk fat and protein, 3.5% FCM and feed efficiency. Supplemental SC had no effect on LPS concentrations in feces (p>0.05) while it trended to reduce (p = 0.07 or 0.207) the concentration in plasma. The results indicate that supplemental SC can increase lactation performance of dairy cattle and has potential for reducing plasma LPS concentration.
INTRODUCTION
As the supply of high quality forage is limited in China, dairy cows are often fed high concentrate diets with low quality forages, putting dairy cows in China at a higher risk of metabolic disease, such as sub-acute ruminal acidosis (SARA), displaced abomasum, ketosis, and fatty liver. Recent research has highlighted the role of high grain feeding in metabolic diseases and has suggested that endotoxin is involved in metabolic diseases (Gozho et al., 2005; Li et al., 2010). It has been shown that high-grain feeding increases rumen and fecal concentrations of free lipopolysaccharide (LPS), which is due to an increase in lysis of gram-negative bacteria (Gozho et al., 2005). Li et al. (2010) reported that high-grain feeding increased fermentation and the production of bacterial toxins in the hindgut of dairy cows. These recent scientific advancements suggest that there may be a correlation between high-grain feeding and fecal LPS and may allow the use of fecal LPS as a diagnostic or management tool for the gut health. On the other hand, enhanced blood LPS concentration may be indicative of health problems such as mastitis or metritis (Pejsak and Tarasiuk, 1989). Therefore, application of blood and fecal LPS assays could be an additional management tool for dairies to monitor the health status of the animals. However, information on blood and fecal LPS concentrations in lactating dairy cows on commercial dairies in China is limited.
Saccharomyces cerevisiae fermentation product (SC), has been shown to improve lactation performance when the rumen was challenged with highly fermentable carbohydrates (Longuski et al., 2009). Stimulating rumen fermentation (Miller-Webster et al., 2002) and stabilizing rumen environment evidenced by a reduction in ruminal LPS concentration under SARA induction condition (Li et al., 2012) indicates that the addition of SC may have a beneficial effect on the cow’s health and performance. Thus, the objectives of the present study were: i) to evaluate the effect of SC on lactation performance; ii) to establish a baseline level of blood and fecal LPS of lactating dairy cows from commercial dairies; iii) to determine the effect of SC on blood and fecal LPS level.
MATERIALS AND METHODS
All experiments were performed in accordance with the guidelines of the Animal Ethics Committee of Jiangsu province (China) and were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University, China.
Animals and diets
Two commercial dairy farms were identified as non-Diamond V users near Nanjing, China. On each farm, 32 cows in early to mid lactation (21 to 140 d of lactation) were selected, blocked by parity (primiparous and multiparous), days in milk (DIM) and somatic cell count (SCC), and then randomly assigned to one of the two treatments, control or SC (Original SC Yeast Culture, Diamond V Mills, Cedar Rapids, Iowa, USA) within block. All cows received the same basal feed. Cows supplemented with SC received 56 g SC/cow/d, top-dressed in the morning feeding for 28 d. Control cows received 56 g/cow/d of grain mixtures (1:1 mixture of corn and soybean meal), top-dressed in the morning feeding for 28 d. The cows were housed in tie stalls with free access to water at all times. Diets were fed as a TMR with ratios of forage to concentrate of 48:52 and 45:55 for Farm 1 and Farm 2, respectively. Cows were fed for ad libitum intake twice daily at 0400 and 1400 h in equal portions. The amount of feed offered was adjusted daily to obtain approximately 10% orts (as-fed basis).
Diets were formulated to meet or exceed the nutrient requirements of a 600-kg lactating cow according to NRC (2001) guidelines. Diet ingredients were analyzed for concentrations of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), calcium (Ca) and phosphorous (P). The DM concentration was determined by drying samples at 105°C for 2 h (AOAC 1990). Methods of Van Soest et al. (1991) were used in analyses of NDF and ADF using heat-stable amylase and sodium sulfite in the case of NDF. The CP (N×6.25) was determined by the method of Krishnamoorthy et al. (1982). The Ca and P content were analyzed according to Official Analytical Chemist (AOAC, 1990) method. Ingredients and nutrient composition of the TMR are presented in Table 1.
Sample collection
Cows were milked three times daily in their stall at approximately 0400, 1200, and 2000 h. Milk yield was recorded daily. Milk samples were collected at every milking on d 0, 8, 15, 22 and 28 of experiment period and were analyzed for protein, fat and lactose by infrared analysis with a Fossmatic-605 (Foss Electric, Hillerod, Denmark). The amounts of TMR offered and refused were measured and recorded daily, and DMI was calculated on d 0, 8, 15, 22 and 28 of experiment period of each period.
On d 0 and 28, fecal grab samples (approximately 50 g) were collected at 0300 h before morning feed delivery and blood was drawn from the tail vein using heparinized vacutainers (Becton Dickinson, NJ, USA) from each cow at 0330 h before morning feeding. Samples were placed in a cooler and delivered to the lab immediately. Blood and fecal LPS concentrations were determined in duplicates per sample.
LPS analysis in fecal samples
About 35 g fecal samples were transferred into a 50 ml centrifuge tube and centrifuged at 13,000×g for 40 min at 4°C. Once centrifuged, all tubes were removed gently from the centrifuge and 5 ml of the supernatant was aspirated into a syringe. Free LPS content in supernatant was determined by the Limulus Amebocyte Lysate (LAL) assay (Xiamen Houshiji, Ltd., Xiamen, China). Pretreated fecal samples were diluted until their LPS concentrations were in the range of 0.1 to 1 endotoxin units (EU)/ml relative to the reference endotoxin (Escherichia coli O111:B4) and assayed as described by Gozho et al. (2005).
LPS analysis in blood plasma samples
As described by Khafipour et al. (2009), the concentration of LPS in the plasma was determined by the LAL assay (Xiamen Houshiji, Ltd., Xiamen, China) with a minimum detection limit of 0.01 EU/ml. Before the LAL assay, samples were pretreated as described by Khafipour et al. (2009). Frozen plasma samples were thawed at 37°C and vortexed. Then, 100 μl of each sample was diluted at least 10-fold with pyrogen-free water. Diluted samples were incubated at 37°C for 30 min, heated at 75°C for 15 min and cooled to room temperature (19°C) for 45 min. The LAL assay was performed using a 96 well microplate according to the manufacturer’s instructions. All of the samples were tested in duplicate, and optical density values were measured using a microplate spectrophotometer (Spectramax 190, Molecular Devices Corporation, CA, USA) at a wavelength of 405 nm. Results were accepted when the intra-assay coefficient of variation was <10%.
Statistical analysis
The 3.5% FCM and feed efficiency were calculated according to the following formulae: 3.5% FCM = (milk kg×0.432)+(fat kg×16.216); Milk efficiency = milk yield/DMI. To test the influence of SC supplementation on dry matter intake, milk protein %, milk fat %, milk yield, milk fat yield, milk protein yield, 3.5% FCM and feed efficiency, the MIXED procedure of SPSS 18.0 (SPSS Inc, Chicago, IL, USA) was performed using diet, DIM and days of experiment as fixed effects and d 0 values as a covariate. Samples collected on different day for the same cow were considered as repeated measures in the analysis of variance (ANOVA). For the LPS data, the general linear model (GLM) procedure of SPSS 18.0 (SPSS Inc, Chicago, IL, USA) was performed with diet and DIM as fixed effects and d 0 values as a covariate. Significance was declared at p≤0.05 and a tendency was considered to exist at 0.05<p<0.10.
RESULTS
Pre-trial production performance and the baseline level of LPS in plasma and feces
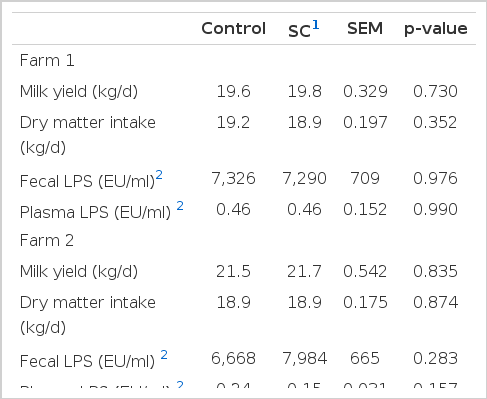
No significant differences (p>0.05) in pre-trial (d 0) milk yield, DMI, and LPS levels in plasma and feces between control and SC were noted on both farms (Table 2). Milk yield and DMI ranged between 19.6 and 21.7 kg/d, and 18.9 and 19.2 kg/d, respectively. Plasma LPS levels ranged from 0.15 to 0.46 EU/ml. Fecal LPS ranged from 6,668 to 7,984 EU/ml.
Effect of SC on production performance
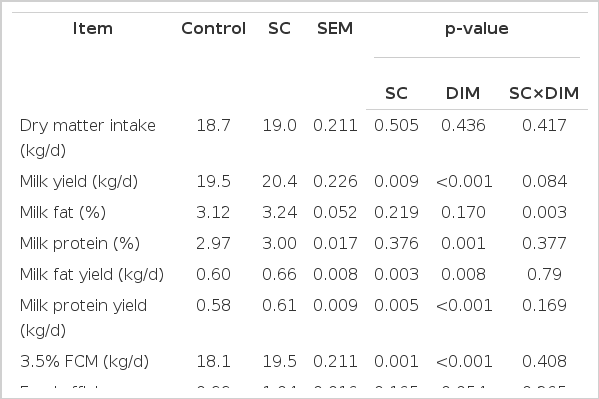
On Farm 1, yield of milk (p = 0.009), milk fat (p = 0.003), milk protein (p = 0.005) and 3.5% FCM (p = 0.001) increased when cows received the SC-supplemented diet (Table 3). No significant changes (p>0.05) were observed on percentage of milk fat and protein and dry matter intake. A significant SC by DIM interaction (p = 0.003) was observed for percentage of milk fat. Effect of SC in milk fat % increased as DIM progresses (Figure 1).
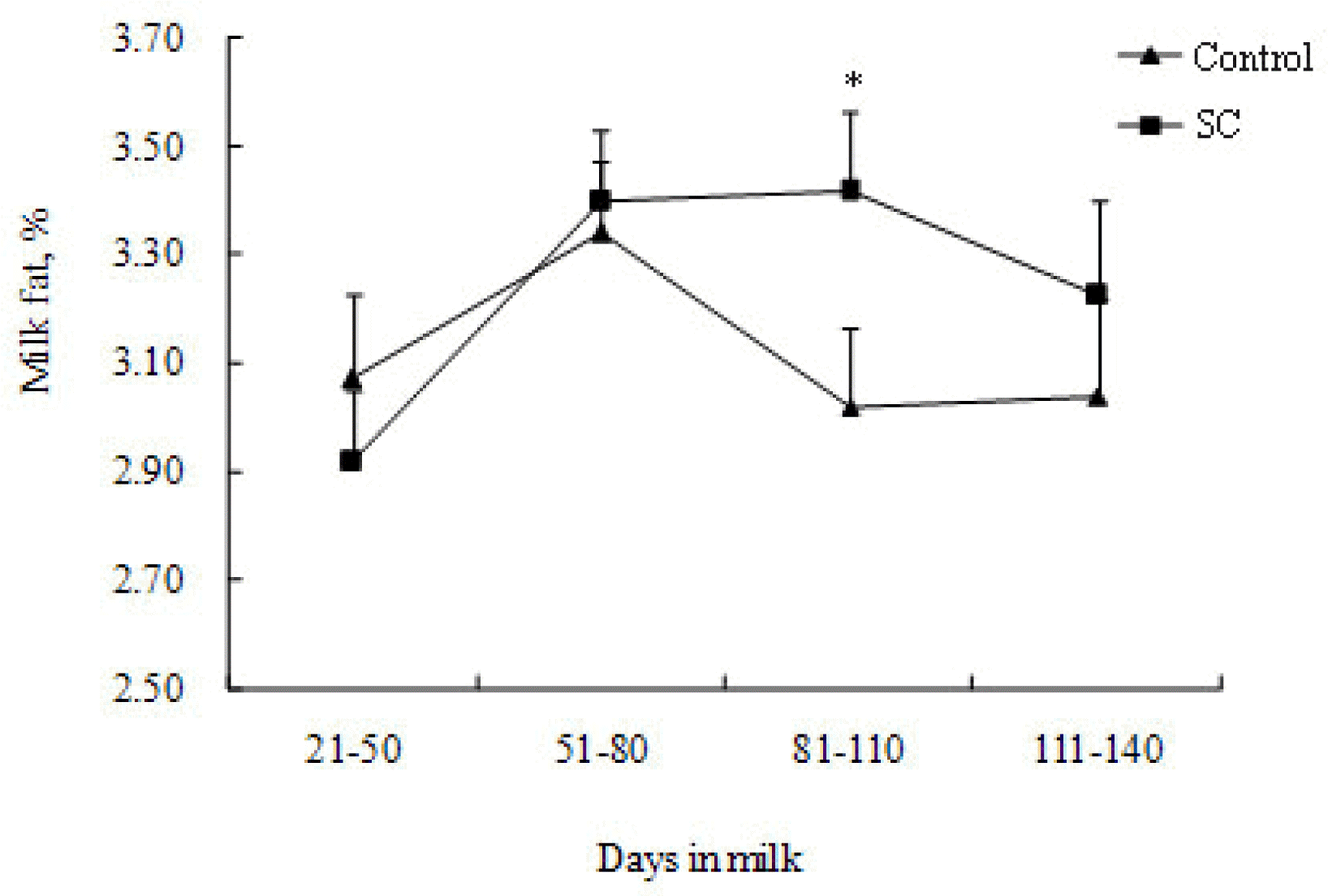
Interaction between SC and DIM on percentage of milk fat (Farm 1). SC was fed at 56 g/cow/d (Diamond V Original XP, Diamond V, Cedar Rapids, Iowa, USA). Note: symbol on the top of standard error bars indicate the significance of the differences between data for control and SC treatment.
On Farm 2, the average daily milk yield (p = 0.017), milk protein % (p<0.001), milk fat % (p = 0.009), milk fat yield (p = 0.002), milk protein yield (p = 0.001), 3.5% FCM (p = 0.002) and feed efficiency (p = 0.011) were significantly increased with SC supplementation (Table 4). Significant SC by DIM interactions were observed in milk yield (p = 0.028), percentage of milk protein (p<0.001) and milk fat (p = 0.049), and milk protein yield (p = 0.030) (Figure 2a, 2b, 2c). Supplementation of SC resulted in an increased milk production (p<0.001) from 111 to 140 DIM. Milk fat (p<0.05) concentrations were greater for cows fed SC from 51 to 110 DIM. Cows from 51 to 140 DIM supplemented with SC had higher percentage of milk protein (p<0.05).

Interaction between SC and DIM on percentage of milk fat (a) and milk protein (b), and milk yield (c) (Farm 2). SC was fed at 56 g/cow/d (Diamond V Original XP, Diamond V, Cedar Rapids, Iowa, USA). Note: symbols on the top of standard error bars indicate the significance of the differences between data for control and SC treatment.
Effect of SC on free LPS concentration in blood and feces
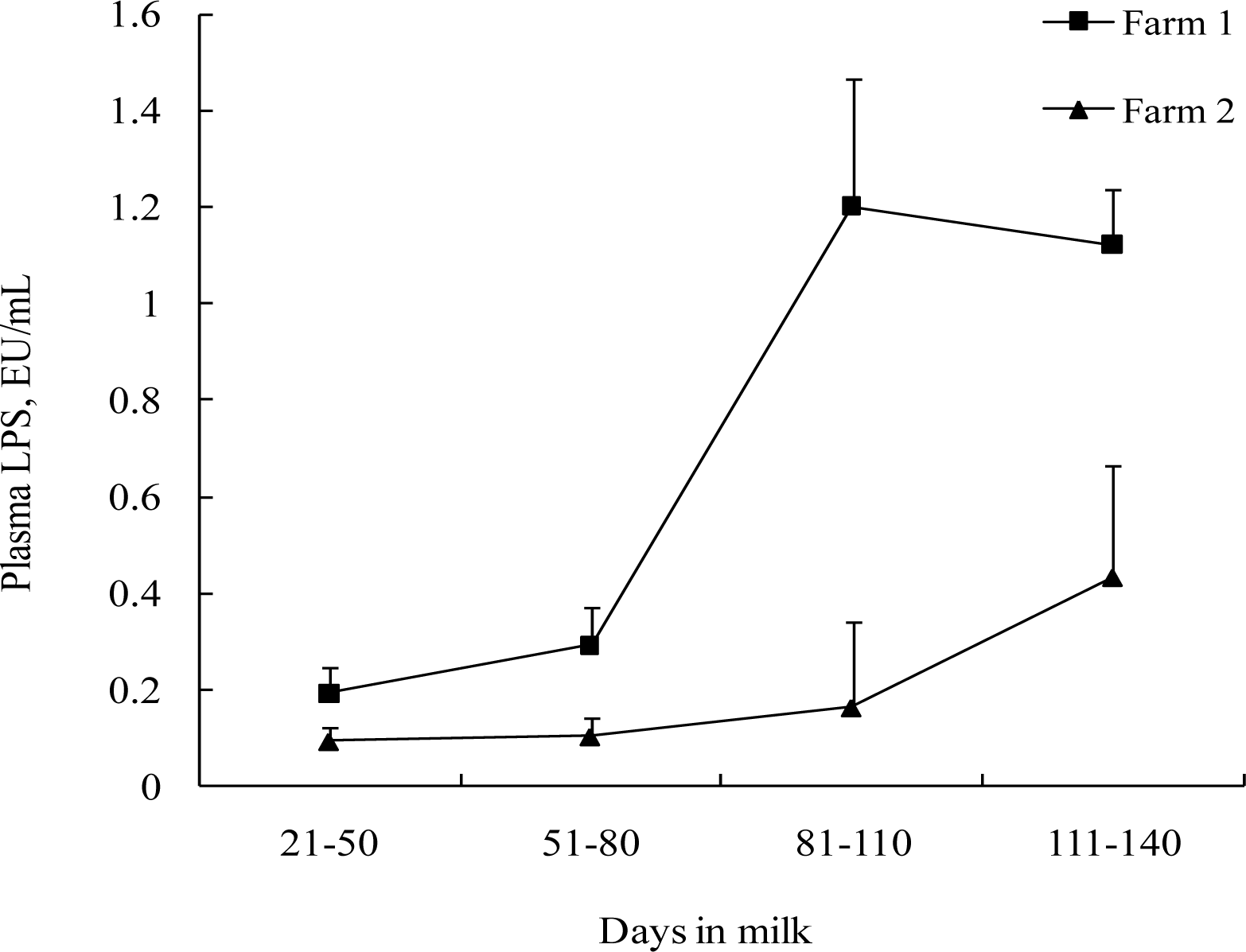
The effects of SC supplementation on free LPS levels in blood and feces are shown in Table 5. Supplementation of SC tended to decrease (p = 0.07) LPS in plasma in Farm 2, with only a numerical reduction (p = 0.207) in Farm 1. Plasma LPS concentration increased (p<0.05) as DIM progresses (Figure 3). Supplementation of SC did not affect (p>0.05) fecal LPS concentrations. Effect of DIM on fecal LPS concentration was not clearly ascertained. There was a significant increase in fecal LPS with increasing DIM on Farm 1 (p<0.001), while no significant change was observed in Farm 2. In addition, there was no significant (p>0.10) SC by DIM interaction for LPS levels in plasma and feces.
DISCUSSION
A recent meta-analysis conducted by Poppy et al. (2012) has shown that SC increases production performance of lactating dairy cows. The increase in milk production estimated for cows fed SC in peer reviewed studies was 1.2 kg/d more milk, 1.6 kg/d more 3.5% FCM or 1.7 kg/d more energy corrected milk. Dry matter intake was estimated to increase by 0.6 kg/d in cows less than 70 DIM and decreased by 0.8 kg/d in cows later in lactation. In the present study, supplementation of SC significantly improved milk yield on both farms. The range of increase in milk production (1.0 kg/d in Farm 1 and 1.4 kg/d in Farm 2) is consistent with the results of the meta-analysis (1.2 kg/d, Poppy et al., 2012). These effects of SC may be due to the stabilizing effect on rumen fermentation, such as increasing lactate-utilizing bacteria (Callaway and Martin, 1997). The stabilized rumen condition allows increased growth and activity of fiber-digesting bacteria (Harrison et al., 1988), resulting in improved fiber digestion (Yoon and Garrett, 1998).
The meta-analysis also reported that SC increased milk fat yield (0.06 kg/d; p = 0.009) and milk protein yield (0.03 kg/d; p = 0.026). However, the paper did not report changes in percentage of milk fat or protein. In the present study, percentage of milk fat was positively affected by SC supplementation (3.12 to 3.24% in Farm 1 and 2.79 to 3.11% in Farm 2). The percentage of milk protein also was positively affected by SC supplementation (2.97 to 3.00% in Farm 1 and 2.97 to 3.08% in Farm 2). These positive effects on percentages of milk fat and protein along with increased milk production resulted in increased milk fat yield (0.06 kg/d in Farm 1 and 0.11 kg/d in Farm 2) and milk protein yield (0.03 kg/d in Farm 1 and 0.06 kg/d in Farm 2) and supports the results seen in the meta-analysis of Poppy et al. (2012).
Average DM intake was approximately 18.8 kg/d in this study. Effect of SC on DM intake is dependent on the status of energy balance of cows. When cows were under negative energy balance (<70 DIM), SC increased DMI. However, under positive energy balance (>70 DIM), SC decreased DMI (Poppy et al., 2012). The present study showed DMI in SC supplemented cows was similar to that of non-supplemented group when averaged for cows from 20 to 140 DIM. However, there was a trend of reducing DMI for cows supplemented with SC compared to control cows as the lactation progressed on Farm 1 but not on Farm 2. Therefore, it was noteworthy that, in the present study, even though the TMR contained 45% to 48% forage, there were not grossly overloaded with concentrates, the SC supplementation still had a significant beneficial effect on lactation performance in both farms. This indicated that the SC supplementation enabled better presentation of the nutritional value of feeds, to increase the concentrate may not be the necessary way to improve the lactation performance considering the health of dairy cows.
Previous research reported that LPS, an endotoxin, could stimulate localized or systemic inflammation via the activation of pattern recognition receptors (Emmanuel et al., 2008). Additionally, LPS and inflammation can regulate intestinal epithelial function by altering integrity, nutrient transport and utilization. The gastrointestinal tract is a large reservoir of both Gram-positive and negative bacteria, of which the Gram-negative bacteria serve as a source of LPS. Recent research has shown that feeding of high concentrate diets is associated with activation of a non-specific acute-phase reaction (APR) in both dairies (Emmanuel et al., 2008; Khafipour et al., 2009) and beef cattle (Ametaj et al., 2009). The reason for the activation of a systemic APR is that translocation of LPS into the systemic circulation stimulates the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 by liver macrophages (Gabay and Kushner, 1999). This results in enhanced secretion of acute phase proteins (APP) like LPS-binding protein (LBP), serum amyloid A (SAA), and C-reactive protein from hepatocytes (Emmanuel et al., 2008).
Rumen pH is believed to play a modulatory role on the release and accumulation of LPS due to its effects on metabolic processes and changes in the cell membrane of rumen bacteria, maintenance of bacterial ecological balances, and on other physiological functions of the rumen (Russell and Rychlik, 2001). Ametaj et al. (2010) observed that a strong negative relationship between preprandial rumen pH and concentration of LPS in the rumen fluid. Thus, rumen pH modifiers could affect the endotoxin concentration. A previous study showed that SC supplementation to cows in early lactation decreased plasma LPS concentration at 20, 40 and 60 d of feeding (Luo et al., 2005). More recently, Li et al. (2012) reported a reduction of ruminal LPS concentration for cows supplemented with yeast culture when cows were subjected to a sub-acute ruminal acidosis induction. In our study, SC supplementation tended to decrease plasma LPS levels. This effect of SC may be partly explained by the stabilizing effect on rumen fermentation as mentioned early.
CONCLUSION
Supplementation of SC to lactating dairy cows significantly improved yield of milk and milk components without affecting DMI, which resulted in improved feed efficiency. Plasma LPS concentrations tended to increase as lactation progressed until 140 DIM. Supplemental SC did not influence LPS concentrations although numerical trend toward reducing the plasma LPS concentrations were noted.
Acknowledgements
This study was supported by funds from Diamond V (Cedar Rapids, IA). The authors thank Weiguo Liu (Department of Animal Science, Anhui Institute of Science and Technology, Anhui) for providing the experimental cows from the commercial dairy farms.