Hepatic transcriptional changes in critical genes for gluconeogenesis following castration of bulls
Article information
Abstract
Objective
This study was performed to understand transcriptional changes in the genes involved in gluconeogenesis and glycolysis pathways following castration of bulls.
Methods
Twenty Korean bulls were weaned at average 3 months of age, and castrated at 6 months. Liver tissues were collected from bulls (n = 10) and steers (n = 10) of Korean cattle, and hepatic gene expression levels were measured using quantitative real-time polymerase chain reaction. We examined hepatic transcription levels of genes encoding enzymes for irreversible reactions in both gluconeogenesis and glycolysis as well as genes encoding enzymes for the utilization of several glucogenic substrates. Correlations between hepatic gene expression and carcass characteristics were performed to understand their associations.
Results
Castration increased the mRNA (3.6 fold; p<0.01) and protein levels (1.4 fold; p< 0.05) of pyruvate carboxylase and mitochondrial phosphoenolpyruvate carboxykinase genes (1.7 fold; p<0.05). Hepatic mRNA levels of genes encoding the glycolysis enzymes were not changed by castration. Castration increased mRNA levels of both lactate dehydrogenase A (1.5 fold; p<0.05) and lactate dehydrogenase B (2.2 fold; p<0.01) genes for lactate utilization. Castration increased mRNA levels of glycerol kinase (2.7 fold; p<0.05) and glycerol-3-phosphate dehydrogenase 1 (1.5 fold; p<0.05) genes for glycerol utilization. Castration also increased mRNA levels of propionyl-CoA carboxylase beta (mitochondrial) (3.5 fold; p<0.01) and acyl-CoA synthetase short chain family member 3 (1.3 fold; p = 0.06) genes for propionate incorporation.
Conclusion
Castration increases transcription levels of critical genes coding for enzymes involved in irreversible gluconeogenesis reactions from pyruvate to glucose and enzymes responsible for incorporation of glucogenic substrates including lactate, glycerol, and propionate. Hepatic gluconeogenic gene expression levels were associated with intramuscular fat deposition.
INTRODUCTION
Glucose metabolism is tightly regulated to meet the energy demands of animals, and significantly contributes to animal growth, as well as the quantity and quality of beef production [1]. Glucose metabolism in adult ruminants is different from that of monogastric animals. In ruminants, large portions of ingested carbohydrates are fermented by anaerobic rumen microbes into short-chain fatty acids (mainly acetate, propionate, and butyrate), resulting in much less glucose absorption from the gastrointestinal tract compared to monogastric animals [2]. Therefore, the glucose requirement of ruminants is largely met through de novo glucose synthesis via gluconeogenesis, which occurs predominantly in the liver [1].
Gluconeogenesis and glycolysis pathways are important for hepatic glucose homeostasis. These are not identical pathways operating in opposite directions, although they share several steps. There are three bypass (irreversible) reactions within the gluconeogenesis and glycolysis pathways. The first bypass reaction in gluconeogenesis is the conversion of pyruvate to phosphoenolpyruvate (PEP), involving the enzymes pyruvate carboxylase (PC) and PEP carboxylase (PCK). The next irreversible reaction is the conversion of fructose 1,6-bisphosphate to glucose 6-phosphate by the enzyme Fructose 1,6-bisphosphatase (FBP1). The third bypass reaction is the conversion of glucose 6-phosphate to glucose by the enzyme G6PC. In the hepatic glycolysis pathway, the first irreversible reaction is the conversion of glucose to glucose 6-phosphate by glucokinase. In the second irreversible reaction, phosphofructokinase-1 (PFKL) is involved in the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate. In the third irreversible reaction, PEP is converted to pyruvate by pyruvate kinase (PKLR).
In ruminants, the important glucogenic substrates of gluconeogenesis are lactate, glycerol, and propionate as well as certain amino acids [3]. Several enzymes are involved in the incorporation of these substrates in the gluconeogenesis pathway [3]: lactate dehydrogenase (LDH) for lactate; glycerol kinase (GK) and glycerol-3-phosphate dehydrogenase (GPD) for glycerol; and acyl-CoA synthetase short-chain family member 3 (ACSS3), propionyl-CoA carboxylase (PCC), methylmalonyl-CoA epimerase (MCEE), and methylmalonyl-CoA mutase (MUT) for propionate. Our previous study showed higher circulating glucose levels in steers than in bulls [4]. This increase may be due in part to changes in the activities of the gluconeogenesis and/or glycolysis pathways following bull castration. However, the molecular events associated with gluconeogenesis and glycolysis pathways in the liver following bull castration remain poorly understood.
Castration is an important method used in the beef cattle industry to improve meat quality, reduce aggressive behavior, and improve herd management. In Korean cattle, beef quality grade (QG) is determined mainly by the amount of marbling [5]. Castration is an efficient way to enhance marbling, resulting in improved beef quality [4], although it decreases growth rate, feed efficiency, dressing percentage, and meat yield [6]. Castration of male rats reduces circulating testosterone levels and enhances hepatic gluconeogenesis [7]. Our previous study revealed a significant reduction in circulating testosterone levels in bulls following castration [4]. Little is currently known about the effects of bull castration on gluconeogenesis processes.
The objective of this study was to elucidate transcriptional changes in the genes involved in the gluconeogenesis and glycolysis pathways following bull castration. We examined whether castration affects the expression of genes encoding enzymes for three bypass (irreversible) reactions in gluconeogenesis and glycolysis from pyruvate to glucose and vice versa. We also determined whether castration affects the expression of genes encoding enzymes responsible for the incorporation of glucogenic substrates (lactate, glycerol, and propionate) into the gluconeogenesis pathway. The associations of gene expression levels with backfat thickness, marbling score (MS), and QG were also examined by correlation analyses.
MATERIALS AND METHODS
Animals, diet, tissue sampling, and carcass characteristics
Liver tissues of 10 bulls and 10 steers from a previous study were used [4]. Briefly, Korean bulls were weaned at a mean age of 3 months, and fed with 30% concentrates/70% roughage until they reached 6 months of age. Bulls were castrated at 6 months. After 6 months of age, bulls and steers were fed with concentrates consisting of 15% crude protein (CP)/71% total digestible nutrients (TDN) until 14 months of age, and 11% CP/73% TDN after 21 months of age. Roughage was offered ad libitum, and the animals had free access to freshwater during the entire experimental period. To study the effects of castration on carcass traits and the associations with gene expression levels, both bulls and steers of conventional slaughter age in Korea beef industry were used. Slaughter ages were 20 months and 28 months for bulls and steers, respectively. Carcass weight was 347 kg and 398 kg for bulls and steers, respectively. We used 10 steers [4] with high MS values to detect differences in liver metabolism between bulls and steers clearly. In our previous study, steers (6.6±0.3) had a 6-fold higher (p< 0.001) MS than bulls (1.1±0.1) [2].
Animals were transported for 4 hours and slaughtered the following day. After undergoing captivate-bolt stunning, the animals were slaughtered in a conventional manner. After slaughter, liver tissue samples were collected immediately, snap-frozen in liquid nitrogen, and stored at −80°C. The carcasses were moved to a cold room (5°C). After 24 hours, the carcasses were graded using the carcass grading system designed by the Korea Institute for Animal Products Quality Evaluation [8].
Carcass characteristics from previous study [4] were used to calculate the correlation coefficients. Briefly, backfat thickness, MS, meat color, fat color, texture, and maturity were determined by an official meat grader. Among these parameters, MS was the main determinant of QG. Five QGs (QG 1++, 1+, 1, 2, and 3) were assigned by meat graders. The beef marbling standard marbling score ranges from 1 (devoid) to 9 (abundant); 8 or 9 was the MS for QG1++, 6 or 7 was the MS for QG1+, 4 or 5 was the MS for QG1, 2 or 3 was the MS for QG2, and 1 was the MS for QG3. Meat color, fat color, texture, and maturity of the exposed longissimus muscle (LM) at the 13th rib interface were used for QG determination [5]. Back fat thickness was evaluated in terms of the thickness of the fat over the LM measured perpendicular to the outside surface at a point two-thirds the length of the rib eye from its chine bone end.
RNA extraction and real-time polymerase chain reaction analysis
Total RNA was isolated from liver tissues using Trizol reagent (Molecular Research Center, Cincinnati, OH, USA) according to the supplier’s protocol. Total RNA concentration and integrity were verified by checking the absorbance at 260 nm and analyzing 2 μL of each sample in 1% agarose gel electrophoresis. RNA quality was also checked by an RNA 6000 Nano LabChip kit and Agilent 2100 BioAnalyzer (Agilent Technologies, Palo Alto, CA, USA).
Total RNA was transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to the supplier’s protocol. All primers were designed using integrated DNA technology, based on published sequences from the National Center for Biotechnology Information (Supplementary Table S1). Gene expression was evaluated using real-time polymerase chain reaction (PCR) analysis in a 25-μL total reaction volume containing 200 ng cDNA, 12.5 μL of SYBR Green RT-PCR Master Mix and 1.25 μL of 10 μM primers. Reactions were performed under thermal cycling condition as follows: 95°C for 15 minutes (initial step) followed by 40 cycles at 94°C for 15 s (denaturation), 55°C for 30 s (annealing), and 72°C for 30 s (extension). Real-time PCR data were normalized using the ΔΔCT method to determine relative fold changes [9], and all data were normalized with ribosomal protein s9 as the housekeeping gene.
Western blot analysis
Samples were homogenized in a Polytron homogenizer for 30 s with cold Pro-PREP protein extraction solution (Intron Biotechnology, Seongnam, Korea), and the homogenized samples were incubated at 4°C for 30 min. Samples were centrifuged (13,000×g, 30 min, 4°C) and the protein content of each supernatant was determined using a BCA protein assay kit (Pierce, Rockfold, IL, USA). Total proteins were prepared for Western blot analysis by boiling in 5× sample buffer (50 mM Tris, 2% sodium dodecyl sulfate, 5% glycerol, and 10% 2-mercaproethanol, pH 6.8). We separated 20 μg of proteins by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 6% polyacrylamide separating gels for PC (130 kDa), with 5% stacking gels. Proteins were transferred into polyvinylidene flouride membranes (BioRad Laboratories, Inc., Hercules, CA, USA) blocked with 1×tris-buffered saline/0.1% Tween 20 and 5% nonfat dried milk, and incubated using commercial primary antibodies (1:200 dilution for antibodies against PC), including goat polyclonal antibody (sc-46228; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The blots were treated with secondary horseradish peroxidase–conjugated anti-goat antibody, and developed using an enhanced chemiluminescence detection kit (Amersham Biosciences, Buckinghamshire, UK). The signal intensity was quantified using image processing scan analysis (ChemiDoc XRS, BioRad, USA) and measured using ImageJ software (NIH, Bethesda, MD, USA). Band densities were normalized with actin content.
Statistical analysis
All data are presented as the mean+standard error of the mean. Statistical differences between bulls and steers were examined using the general linear model procedure in the SAS software 9.1 (SAS Institute, Cary, NC, USA). Correlations of gene expression levels with back fat thickness, MS, and QG were calculated using the CORR procedure in SAS.
RESULTS
Expression of genes for the gluconeogenesis pathway from pyruvate to glucose
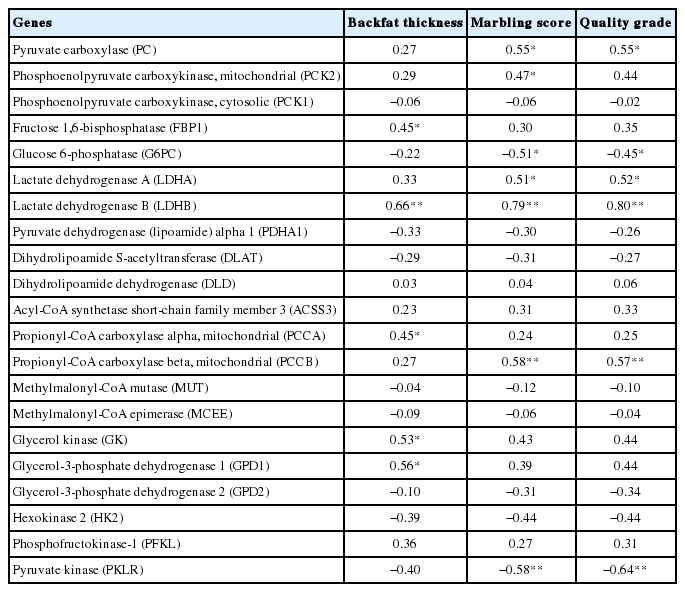
In this study, we examined changes in the expression of genes encoding enzymes responsible for three bypass reactions in the gluconeogenesis pathway following castration. Pyruvate carboxylase is the regulatory enzyme of gluconeogenesis, and it catalyzes the conversion of pyruvate to oxaloacetate (OAA). We found higher (3.6 fold; p<0.01) PC mRNA levels in steers than in bulls (Figure 1). The PC protein levels were examined by Western blot. Steers exhibited higher (p<0.05) PC protein levels in the liver than did bulls, reflecting the transcriptional activity trend.
Hepatic expression levels of genes for gluconeogenesis from glucose to pyruvate in Korean cattle. mRNA levels (n = 10) were determined using real-time polymerase chain reaction and normalized with a control ribosomal protein s9 gene. mRNA levels in bulls were normalized to 1.0. Protein levels (n = 4) were determined by Western blot analysis and normalized with actin levels. Values are expressed as the mean+standard error. A–B Means with different superscripts were different at p<0.01 between bulls and steers. a–b Means with different superscripts were different at p<0.05 between bulls and steers. PC, pyruvate carboxylase; PCK2, mitochondrial phosphoenolpyruvate carboxykinase; PCK1, cytosolic phosphoenolpyruvate carboxykinase; FBP1, fructose 1,6-bisphosphatase; G6PC, glucose 6-phosphatase.
Phosphoenolpyruvate carboxykinase catalyzes the decarboxylation of OAA to produce PEP. In gluconeogenesis, the conversion of OAA into PEP by PCK can occur in both the cytosol and mitochondria. One of two isoforms of PCK, the cytosolic form (PCK1) and the mitochondrial form (PCK2), works in each compartment. In the mitochondria, OAA can be converted into PEP by PCK2 and transported to the cytoplasm, where it is converted into glucose via the remaining gluconeogenic enzymes. In this study, we found higher (1.7 fold; p<0.05) PCK2 mRNA levels in steers than in bulls (Figure 1). In this study, castration did not affect (p>0.05) PCK1 mRNA levels.
In this study, the mRNA levels of FBP1 and glucose 6-phosphatase (G6PC) remained unchanged (p>0.05) following cas-tration (Figure 1). Al-Trad et al [10] also reported no significant correlation between glucose release from the gluconeogenesis pathway and the change in FBP1 and G6PC mRNA abundance, which was mainly regulated post-transcriptionally.
Expression of genes for the incorporation of glucogenic substrates in the gluconeogenesis pathway
In this study, we examined changes in the hepatic expression levels of genes encoding enzymes for the incorporation of glucogenic substrates (lactate, glycerol, and propionate) following castration (Figure 2). Lactate is first converted to pyruvate by LDH in the cytosol. In this study, castration increased hepatic expression levels of both LDHA (muscle type; 1.5 fold; p<0.05) and LDHB (liver type; 2.2 fold; p<0.01) genes.

Hepatic mRNA levels of genes for glucogenic substrate incorporation in gluconeogenesis pathway in Korean cattle. mRNA levels were determined by real-time polymerase chain reaction and normalized with a control ribosomal protein s9 gene. mRNA levels in bulls were normalized to 1.0. Values are expressed as the mean+standard error (n = 10). A–B Means with different superscripts were different at p<0.01 between bulls and steers. a–b Means with different superscripts were different at p<0.05 between bulls and steers. LDHA, lactate dehydrogenase A; LDHB, lactate dehydrogenase B; PDHA1, pyruvate dehydrogenase (lipoamide) alpha 1; DLAT, dihydrolipoamide S-acetyltransferase; DLD, dihydrolipoamide dehydrogenase; GK, glycerol kinase; GPD1, glycerol-3-phosphate dehydrogenase 1; GPD2, glycerol-3-phosphate dehydrogenase 2; ACSS3, acyl CoA synthetase short chain family member 3; PCCA, propionyl CoA carboxylase alpha; PCCB, propionyl CoA carboxylase beta; MCEE, methylmalonyl CoA epimerase; MUT, methylmalonyl CoA mutase.
Pyruvate can either be converted to OAA by PC or reduced to acetyl-CoA by the pyruvate dehydrogenase complex. The complex consists of three enzymes: pyruvate dehydrogenase (lipoamide) alpha 1 (PDHA1), dihydrolipoamide S-acetyltransferase (DLAT), and dihydrolipoamide dehydrogenase (DLD). In this study, hepatic mRNA levels of PDHA1, DLAT, and DLD genes did not differ (p>0.05) between bulls and steers. Our study demonstrated that castration did not change acetyl-CoA generation from pyruvate.
Glycerol can be utilized as a glucogenic precursor. Glycerol is converted to glycerol-3-phosphate by GK in the liver. Glycerol-3-phosphate is then oxidized to dihydroxyacetone phosphate by GPD and utilized for gluconeogenesis. Two isoforms (GPD1 in the cytosol and GPD2 in the mitochondria) are present; both enzymes catalyze the reversible conversion of glycerol-3-phosphate to dihydroxyacetone phosphate. We examined hepatic transcript levels of genes for the incorporation of glycerol into the gluconeogenesis pathway in bulls and steers. Castration increased mRNA levels of the GK (2.7 fold; p<0.05) and GPD1 (1.5 fold; p<0.05) genes (Figure 2), but did not affect hepatic expression of the GPD2 gene.
Propionate is the major substrate for gluconeogenesis in ruminants [3]. Castration affected the expression of several genes for the incorporation of propionate into the gluconeogenesis pathway (Figure 2). ACSS3 plays a role in the activation of acetate to specific metabolic fate, and has also a high activity to activate propionate [11]. In mitochondria, ACSS3 catalyzes the conversion of propionate to propionyl-CoA at the first step of the propionate-originated gluconeogenesis pathway [12]. In this study, steers exhibited higher (p = 0.06) ACSS3 mRNA levels than did bulls (Figure 2). Propionyl-CoA is then converted by mitochondrial PCC to methylmalonyl-CoA, which is converted to succinyl-CoA by MCEE and MUT. Succinyl CoA is subsequently incorporated into part of the tricarboxylic acid cycle to generate OAA [3]. Mitochondrial PCC is composed of two subunits, alpha (PCCA) and beta (PCCB), which are encoded by two separate genes [13]. In this study, castration increased (p<0.01) mRNA levels of PCCB, although it did not affect (p>0.05) PCCA mRNA levels (Figure 2). Castration did not affect (p>0.05) mRNA levels of the MCEE and MUT genes (Figure 2).
Expression levels of glucose transport and glycolysis genes
We have examined the expression of genes for hepatic glucose transporter (solute carrier family 2 member 2 [SLC2A2]) and glycolysis. The SLC2A2 gene is a major glucose transporter in the cattle liver [14]. We found no difference (p>0.05) in SLC2A2 mRNA levels between bulls and steers (Figure 3). The mRNA levels of glycolysis genes encoding enzymes hexokinase-2 and PFKL did not differ (p>0.05) between bulls and steers. Our study indicates that castration does not affect the transcriptional activities of glucose uptake and upstream of the glycolysis pathway in the liver.

Hepatic mRNA levels of glucose transporter and glycolysis genes in Korean cattle bulls and steers. mRNA levels were determined by real-time polymerase chain reaction and normalized with a control ribosomal protein s9 gene. mRNA levels in bulls were normalized to 1.0. Values are expressed as the mean+standard error (n = 10). A–B Means with different superscripts were different at p<0.01 between bulls and steers. SLC2A2, solute carrier family 2 member 2; HK2, hexokinase 2; PFKL, phosphofructokinase-1; PKLR, pyruvate kinase.
Pyruvate kinase catalyzes the transfer of phosphate from PEP to adenosine diphosphate to produce adenosine triphosphate (ATP) and pyruvate. In this study, hepatic mRNA levels of the PKLR gene were lower (p<0.01) in steers than in bulls. This may indicate lower PEP conversion for ATP production following castration.
Correlation coefficient data
We analyzed the relationships of hepatic gene expression levels with backfat thickness, MS, and QG using pooled steers and bulls. Several significant correlations were observed (Table 1). Backfat thickness showed positive correlations with the hepatic mRNA levels of genes for gluconeogenesis, including FBP1 (r = 0.45, p<0.05), LDHB (r = 0.66, p<0.01), PCCA (r = 0.45, p< 0.05), GK (r = 0.53, p<0.05), and GPD1 (r = 0.56, p<0.05). MS and/or QG were positively correlated with the hepatic mRNA levels of PC (r = 0.55, p<0.05; r = 0.55, p<0.05), PCK2 (MS r = 0.47, p<0.05), LDHA (r = 0.51, p<0.05; r = 0.52; p<0.05), LDHB (r = 0.79, p<0.01; r = 0.80, p<0.01), and PCCB (r = 0.58, p<0.01; r = 0.57, p<0.01) genes. Negative correlations of MS and QG with hepatic mRNA levels of the G6PC (r = −0.51, p<0.05; r = −0.45, p< 0.05) and PKLR (r = −0.58, p<0.01; r = −0.64, p<0.01) genes were observed.
DISCUSSION
Changes in expression of genes for the gluconeogenesis pathway from pyruvate to glucose following bull castration
Glucose is the major fuel for metabolism in most body tissues. Hepatic glucose homeostasis is determined by the rates of glucose uptake and glucose production. In ruminants, glucose requirements are largely met through gluconeogenesis, which occurs predominantly in the liver. There are three bypass reactions of gluconeogenesis pathway: conversion of pyruvate to PEP by enzymes PC and PCK, conversion of fructose 1,6-bisphosphate to glucose 6-phosphate by FBP1, and conversion of glucose 6-phosphate to glucose by G6PC. In this study, we found that castration increased the expression of PC mRNA and protein levels. Upregulation of PC gene expression may contribute to increased OAA production and subsequent activation of the gluconeogenesis pathway following castration.
In this study, we found that castration upregulated PCK2 gene expression. The PCK2 reaction predominates when lactate is the glucogenic precursor [3]. Upregulation of PCK2 gene expression may indicate that castration increases utilization of lactate as a glucogenic precursor in the gluconeogenesis pathway. Castration of male animals reduces the concentration of circulating androgen. In a rat study, castration was found to reduce circulating testosterone levels and increase body fat mass [7]. In our previous study, castration also decreased testosterone levels and increased intramuscular fat deposition in beef cattle [4]. Insulin is known to suppress lipolysis and gluconeogenesis [15]. Castration alone [7] or a combination of castration and a high-fat diet [16] induced insulin resistance in the liver and other peripheral tissues, such as adipose and muscle tissue, in rodent studies. The authors suggested that increased gluconeogenic Pck gene expression is associated with hepatic insulin resistance, which was induced by androgen deficiency under a combination of castration and feeding a high-fat diet. Thus, the increased hepatic PCK2 transcription levels observed in this study may be also associated with insulin resistance caused by testosterone deficiency following castration. In the cytosol, OAA can also be converted into PEP by PCK1. This pathway is predominant when an amino acid such as alanine is the glucogenic precursor. The mitochondrial membrane possesses no OAA transporter. Thus, OAA is reduced to malate at the expense of NADH, and malate is then transported to the cytosol where it is reoxidized to OAA with the generation of cytosolic NADH. The cytosolic OAA is converted into PEP to enter the gluconeogenesis pathway. In this study, mRNA levels of PCK1 gene did not differ between bulls and steers. It has been proposed that PCK1 is required for gluconeogenesis from amino acids, whereas PCK2 is better suited for gluconeogenesis from lactate [17]. Thus, our study implies that castration may increase gluconeogenesis by using lactate as glucogenic substrate with no change in amino acid availability for gluconeogenesis.
Changes in expression of genes for the incorporation of glucogenic substrates in the gluconeogenesis pathway following castration
In ruminants, the major glucogenic precursors of gluconeogenesis in the liver are lactate, propionate, glycerol, and amino acids. We found that castration increased hepatic gene expression levels of both LDHA and LDHB, which are involved in conversion of lactate to pyruvate. Lactate can be produced endogenously from the anaerobic glycolysis pathway, mainly in skeletal muscle, but also in other tissues including the brain, gastrointestinal tract, renal medulla, adipose tissue, skin, and erythrocytes [18]. Kuhla et al [19] suggested that increased lactate production in skeletal muscle might serve as a substrate for hepatic gluconeogenesis during the adaptation period in the early lactation of dairy cows. Several studies have reported that lactate metabolism and hepatic lactate uptake are influenced by insulin action. Insulin decreases hepatic lactate uptake in rats [20]. Insulin resistance in obese patients is associated with elevated circulating basal lactate levels in humans [21]. Insulin resistance in obese Zucker rats was also associated with higher circulating lactating concentrations and impaired lactate metabolism in skeletal muscle compared with normal Wistar rats [22]. As described above, several studies have shown that castration induces insulin resistance in the skeletal muscle of male rats [7,16]. Therefore, castration may induce insulin resistance, which limits glucose utilization in skeletal muscle and subsequently increases its lactate output, contributing to increased hepatic lactate uptake. Increased hepatic lactate uptake following castration may in part contribute to increased hepatic gluconeogenic activity through the activation of LDH expression. After pyruvate is transported into the mitochondria, it is converted to OAA by PC, as described above. Our findings on the upregulation of PC and PCK2 gene expression following castration may be due to the increase in lactate availability for the glucogenic substrate. In a rat study, Stark et al [23] found that Pck2 has a direct role in gluconeogenesis from a lactate substrate.
In addition to endogenous lactate production from peripheral tissues, lactate can also be produced in the rumen of ruminant animals by bacterial fermentation if they are fed high amounts of concentrate diet, including starch, and if ruminal pH is relatively low [3]. This lactate can also be used as a gluconeogenesis substrate. In the conventional Korean beef cattle feeding system, steers consume a higher concentrate diet than do bulls during the finishing period to increase MS [24]. In steers, this may induce higher lactate production, which may be used as a glucogenic substrate. Further study is warranted to understand the link between hepatic gluconeogenesis metabolism and lactate production in the skeletal muscle and rumen of cattle.
We found that castration increased gene expression for GK and GPD1, which are involved in the incorporation of glycerol into the gluconeogenesis pathway. This result indicates that castration may increase the utilization of glycerol as a glucogenic source. In a mouse study, a deficiency of Gpd1 activity inhibited the use of glycerol for gluconeogenesis [25]. In mouse and rat studies, insulin resistance has induced lipolysis, resulting in increased generation of fatty acid and glycerol production, which caused hepatic increases in acetyl-CoA production and glycerol input [26]. Adeva-Andany et al [27] reported positive correlation of the rates of gluconeogenesis and fatty acid oxidation. Increased hepatic fatty acid oxidation activates gluconeogenesis, in part, due to increased production of acetyl-CoA, which is an activator of PC [28]. Our previous study showed that castration increases fatty acid oxidation in skeletal muscle of Korean cattle [29]. Perry et al [26] suggested that allosteric regulation of PC activity by hepatic acetyl-CoA is an important factor regulating hepatic glucose production. In their study, insulin resistance increased gluconeogenesis through increased PC activation by acetyl-CoA and increased utilization of glycerol as a glucogenic source. Xia et al [7] also reported that castration-induced testosterone deficiency decreased insulin sensitivity and that it increased hepatic gluconeogenesis, especially from a glycerol substrate. Thus, our observation that increased transcription levels of genes encoding PC, GK, and GPD1 for the utilization of glycerol as a glucogenic substrate may be also associated with insulin resistance caused by testosterone deficiency following castration. Further study is needed to verify whether castration increases hepatic fatty acid oxidation, thus increasing production of acetyl-CoA and glycerol, contributing to activation of the gluconeogenesis pathway.
Overall, the current study showed that the combined effects of bull castration and a high-concentration diet during the fattening period might cause insulin resistance, resulting in increased lipolysis and subsequent increased production of lactate, glycerol, and acetyl-CoA. These increases then may activate several enzymes (LDH, PC, PCK2, GK, and GPD1) responsible for gluconeogenesis.
Collectively, our results indicate that castration increases transcriptional activities of the hepatic gluconeogenesis pathway from propionate through the activation of ACCS3 and PCCB gene expression. In ruminants, feeding a higher-concentrate produces relatively high propionate levels in the rumen [30]. In Korea, steers are typically fed a higher-concentrate during the late fattening period than bulls are, probably causing higher propionate production in steers than in bulls [31]. Thus, increased transcriptional activities of propionate utilization for gluconeogenesis precursors following castration may in part be due to the consumption of a high-concentrate during the late fattening period in steers.
Gluconeogenesis from propionate is less responsive than other substrates to changes in insulin concentration [32]. When lactate and glucogenic amino acids are more available for other synthetic processes, propionate could preferentially be used for gluconeogenesis [2,32], increasing the proportion of glucose derived from propionate. Our results indicate that expression levels of genes encoding enzymes for the gluconeogenesis pathway using several substrates including lactate, glycerol, and propionate are upregulated following castration.
In this study, steers showed the higher hepatic mRNA levels of the PKLR gene than bulls indicating lower PEP conversion for ATP production following castration. In a mouse study, insulin resistance in the liver led to a decrease in the expression of Pklr [33]. As described above, we found that castration increased the mRNA levels of the LDH gene, which is responsible for gluconeogenesis from lactate. Thus, our study demonstrates the shift of PEP utilization from energy (ATP) production to lactate utilization for hepatic gluconeogenesis.
Significant correlation between hepatic gene expression levels and backfat thickness, marbling score, and quality grade
By correlation analysis, we found significant correlations of expression levels of several genes including FBP1, LDHB, PCCA, GK, and GPD1 with backfat thickness. The QG of Korean cattle beef is determined mainly by MS. We also found that hepatic mRNA levels of PC, PCK2, LDHA, LDHB, and PCCB genes were positively correlated with the MS and/or QG. Insulin resistance increased hepatic glucose production; however, it was associated with diminished glucose utilization in muscle and enhanced utilization in white adipose tissue [34]. Prior and Scott [35] suggested that increased availability of glucogenic precursors (lactate and propionate) or glucose may be responsible for the induction of lipogenesis in steer fat tissues. Therefore, significant correlations of glucogenic gene expression with carcass traits may support that increased incorporation of glucogenic substrates from lactate and glycerol or propionate enhance hepatic gluconeogenesis, which contributes to glucose utilization in adipose tissue, resulting in increased backfat thickness and intramuscular fat deposition, and thus MS and QG following castration. Some genes for gluconeogenesis may therefore be used as genetic markers to predict backfat thickness, MS, and QG.
In addition, castration may change the myofiber composition and size in the LM. Castration decreased the type IIA myofiber (fast-twitch oxidative) and increased the type IIB myofiber (fast-twitch glycolytic) in LM [36]. Increased proportion of type IIB muscle fibers has been found to correlate with insulin resistance, which may alter the metabolism and/or storage of lipids in the muscle, partly contributing to increased intramuscular fat deposition following castration.
Our results reveal that castration of bulls upregulates the transcriptional levels of several genes involved in gluconeogenesis, including PCCB, PC, PCK2, LDHA and LDHB, GK, and GPD1 (Figure 4). These results demonstrate that castration may increase gluconeogenesis pathways from several substrates, including lactate, propionate, and glycerol. The castration of male animals significantly reduces circulating androgen concentrations, and androgen deficiency often induces insulin resistance. Insulin resistance is known to increase gluconeogenesis, especially from lactate and glycerol. In contrast, gluconeogenesis from propionate is known to be less responsive to insulin. Overall, castration may increase gluconeogenesis through both insulin-dependent (from lactate and glycerol) and insulin-independent (from pyruvate) pathways in beef cattle. We found positive correlations of gluconeogenic gene expression in the liver with backfat thickness, MS, and QG. These results imply that increased hepatic gluconeogenic gene expression following castration is associated with increased backfat thickness and intramuscular fat deposition, and thus QG.
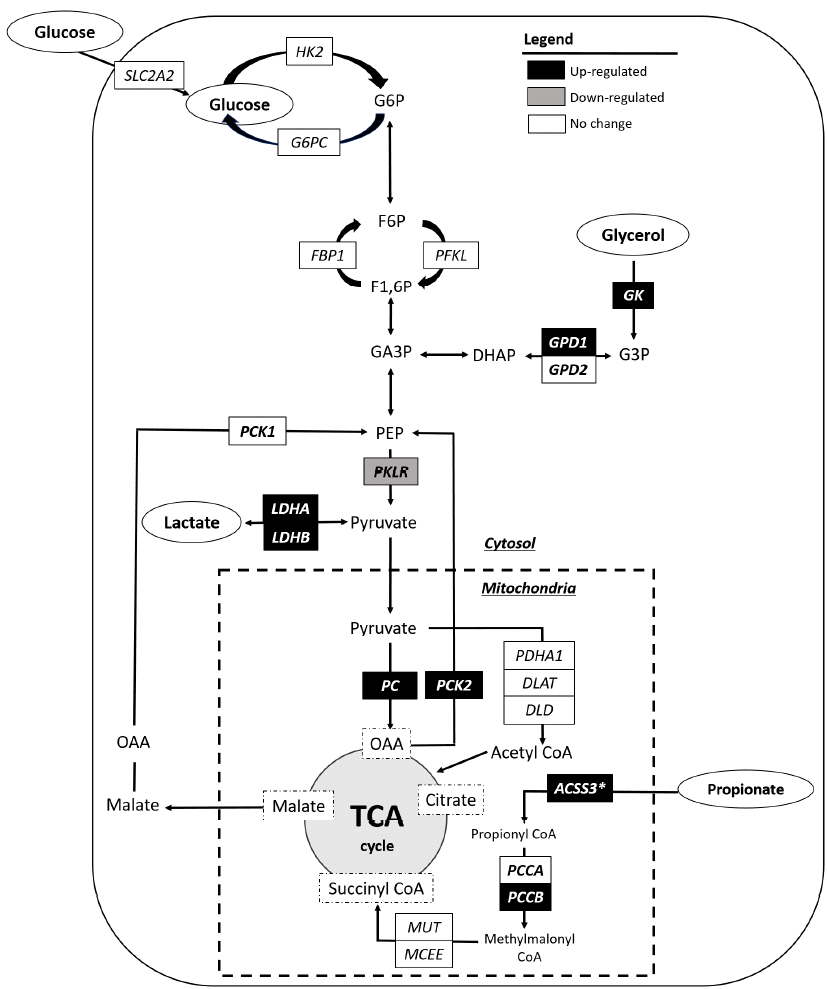
Changes in the hepatic expression levels of genes for gluconeogenesis pathway following castration of bulls. Castration upregulated the mRNA levels of several gluconeogenesis genes, including PCCB, ACSS3* (p = 0.06), PC, PCK2, LDHA, and LDHB, GK, and GPD1, demonstrating that castration increases the transcriptional activities of hepatic gluconeogenesis from several glucogenic substrates (propionate, lactate, and glycerol). ACSS3, acyl-CoA synthetase short-chain family member 3; DLAT, dihyrolipoamide S-acetyltransferase; DLD, dihydrolipoamide dehydrogenase; FBP1, fructose 1,6-bisphosphate; G6PC, glucose 6-phosphatase; GK, glycerol kinase; GPD1, glycerol-3-phosphate dehydrogenase-1; GPD2, glycerol-3-phosphate dehydrogenase-2; HK2, hexokinase 2; LDHA, lactate dehydrogenase A; LDHB, lactate dehydrogenase B; MCEE, methymalonyl CoA epimerase; MUT, methylmalonyl CoA mutase; PC, pyruvate carboxylase; PCCB, propionyl CoA carboxylase beta; PCCA, propionyl CoA carboxylase alpha; PCK1, cytosolic phosphoenolpyruvate carboxykinase; PCK2, mitochondrial phosphoenolpyruvate carboxykinase; PDHA1, pyruvate dehydrogenase (lipoamide) alpha 1, cytosolic; PFKL, phosphofructokinase-1; PKLR, pyruvate kinase; SLC2A2, solute carrier family 2 member 2; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6P, fructose 1,6-bisphosphate; GA3P, glyceraldehyde-3-phosphate; DHAP, dihydroxyacetone phosphate; G3P, glycerol-3-phosphate; PEP, phosphoenolpyruvate; OAA, oxaloacetate; TCA, tricarboxylic acid cycle.
Supplementary Data
ACKNOWLEDGMENTS
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2017R1A2B4003207), and Next-Generation BioGreen 21 Program (PJ01114001) funded by Rural Development Administration, Republic of Korea. The first author received a Ph.D. scholarship from the Directorate General of Resources for Research, Technology, and Higher Education of the Republic of Indonesia.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.