Genetic association of swine leukocyte antigen class II haplotypes and body weight in Microminipigs
Article information
Abstract
Objective
Microminipigs are a novel animal model with extensive applications in laboratory studies owing, in part, to their extremely small body sizes. In this study, the relationship between swine leukocyte antigen (SLA) class II haplotype and body weight was evaluated in the Microminipig population.
Methods
A total of 1,900 haplotypes, covering SLA class II haplotypes Lr−0.7, Lr−0.23, Lr−0.17, Lr−0.37, Lr−0.16, Lr−0.11, Lr−0.13, and Lr−0.18, were analyzed in 950 piglets. Birth weights and weights on postnatal day 50 were examined in piglets with eight different SLA class II haplotypes.
Results
The mean birth weight of piglets with the Lr−0.23 haplotype (0.415 kg, n = 702) was significantly lower than that of piglets with Lr−0.17 (0.445 kg, n = 328) and Lr−0.37 (0.438 kg, n = 383) haplotypes. At postnatal day 50, the mean body weight of piglets with the Lr−0.23 haplotype (3.14 kg) was significantly lower than that of piglets with the Lr−0.13 haplotype (3.46 kg, p<0.01). There were no significant differences in daily gains (DGs) among the eight haplotypes. However, piglets with the Lr−0.11 and −0.18 haplotype combination or any heterozygous haplotype combinations containing Lr−0.23 had significantly lower DGs than those of piglets with the Lr−0.18, 0.37 haplotype combination.
Conclusion
Piglets with the Lr−0.23 haplotype had relatively low body weights at birth and on postnatal day 50 and slightly lower DGs than those of piglets with other haplotypes. Therefore, the Lr−0.23 SLA class II haplotype may be a suitable marker for the selective breeding of Microminipigs with small body sizes.
INTRODUCTION
Microminipigs were registered with the Japanese Ministry of Agriculture, Forestry and Fisheries as a new pig line [1]. They have an extremely small body size [1]; a 6-month-old mature Microminipig is 7 to 8 kg, comparable to the size of a mature beagle [1,2]. Their small body size provides many advantages for laboratory use, including ease of handling, small rearing spaces, low feedstuff cost, low doses of test substances, and a decreased environmental load.
The swine major histocompatibility complex, known as the swine leukocyte antigen (SLA), is a gene-dense region containing many important immune-related genes [3,4]. Associations between specific SLA haplotypes and antibody responses to proteins and vaccine antigens influence porcine immune responses to infectious agents and vaccinations [5,6]. Furthermore, SLA haplotypes influence economical swine traits, including growth, body weight, backfat thickness, and carcass composition [4,7–11], although SLA genes themselves are not directly responsible for these traits [12].
In Microminipigs, 11 SLA class I and II haplotypes, including three recombinant haplotypes, have been identified [13]. Accordingly, there is sufficient variation for the analysis of associations between SLA haplotypes and various genetic traits. Here, we examined associations between SLA class II haplotypes and body weight, an important economical trait in Microminipigs.
MATERIALS AND METHODS
This study was performed according to the regulations of Fuji Micra Inc. Randomly selected Microminipigs (n = 950), born between March 2009 and August 2015, with available body weight and SLA class II haplotype data were included. SLA class II-DRB1 and DQB1 alleles were assigned by a low-resolution SLA genotyping technique using polymerase chain reaction-sequence specific primers (PCR-SSP) [14]. SLA class II haplotypes were assigned from the DRB1 and DQB1 alleles based on eight previously identified SLA class II haplotypes, i.e., Lr−0.7, Lr−0.23, Lr−0.17, Lr−0.37, Lr−0.16, Lr−0.11, Lr−0.13, and Lr−0.18, in the Microminipig population [13].
Birth weights were measured immediately after delivery. Body weights at postnatal day 50 were estimated. The daily weight gains (DGs) were calculated from the body weights at birth and at postnatal day 50. Body weights and DGs were compared among the eight SLA haplotypes (n = 1,900), among piglets with homozygous or heterozygous haplotypes containing Lr−0.23 (n = 578), and among piglets with twenty common homozygous or heterozygous haplotypes detected in over 10 individuals in the population (Supplementary Table S1).
The mean body weights of male Microminipigs and domesti cated pigs were slightly higher those of females (data not shown). The numbers of male and female piglets were almost the same for each SLA class II haplotype; thus, we analyzed the weights of male and female piglets, without distinction.
Differences in mean body weights with respect to SLA haplo type were evaluated by analysis of variance and multiple comparison analyses (Tukey–Kramer or Dunnett’s method). Data are expressed as means±standard error, and p-values of less than 0.05 were considered significant.
RESULTS
Of the 950 Microminipig samples, the most frequently observed haplotype was Lr−0.23 (n = 702, 36.9%), followed by Lr−0.37 (n = 383, 20.2%) and Lr−0.17 (n = 328, 17.3%). The two least frequent haplotypes were Lr−0.7 (n = 15, 0.8%) and Lr−0.13 (n = 73, 3.8%). The mean birth weight of piglets with the Lr−0.23 haplotype (n = 702, 0.415 kg) was significantly lower than that of piglets with Lr−0.17 (0.445 kg, n = 328, p<0.01) and Lr−0.37 (0.438 kg, n = 383, p<0.05) haplotypes (Figure 1). Additionally, piglets with Lr−0.13 had a mean birth weight of 0.445 kg (n = 73), which was slightly, but not significantly, higher than that of piglets with Lr−0.23. Furthermore, the mean body weight of Lr−0.23 homozygous piglets was considerably lower than that of piglets heterozygous for Lr−0.23 and seven other haplotypes (Figure 2). Additionally, the mean body weight of homozygous piglets with the Lr−0.23 haplotype was significantly lower (p<0.05) than that of Lr−0.13, −0.23 heterozygous piglets.
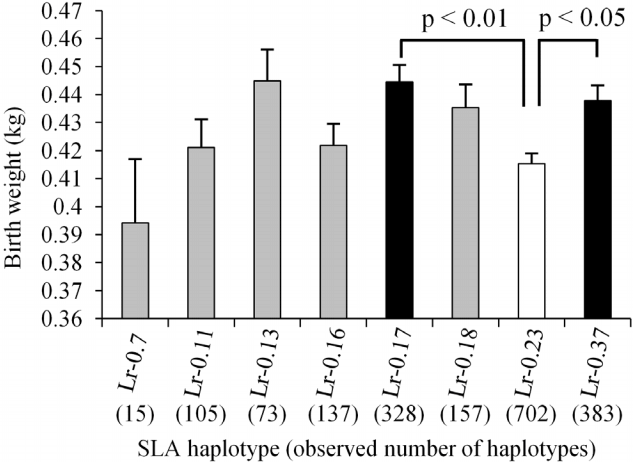
Comparison of birth weights of Microminipigs with eight different swine leukocyte antigen (SLA) class II haplotypes. Columns and bars indicate means and standard error, respectively. The number of haplotypes was counted as two in homozygous individuals. White and black columns represent the lower (Lr−0.23) and higher mean values (Lr−0.17 and Lr−0.37, respectively) with significant differences among haplotypes, respectively. Gray columns represent mean values that did not differ significantly from those of other haplotypes.
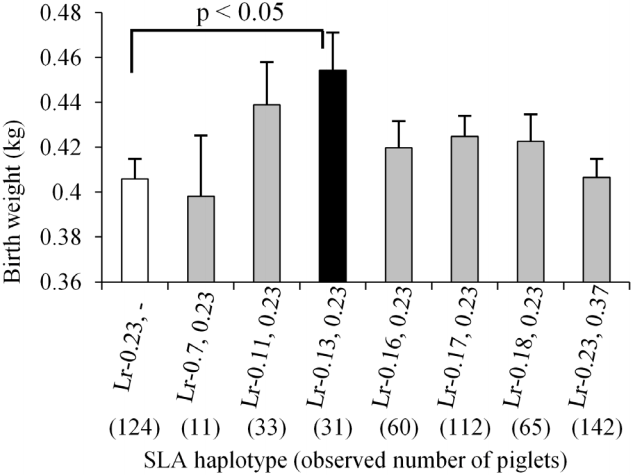
Comparison of birth weights among Lr−0.23 homozygous and heterozygous piglets. Columns and bars indicate means and standard error, respectively. White and black columns represent the lower and higher mean values of Lr−0.23, and Lr−0.13, 0.23, respectively, with significant differences among haplotypes. Gray columns represent mean values that did not differ significantly from those of other haplotypes.
The mean birth weights were compared among homozygous and/or heterozygous piglets with different SLA class II haplotypes (Supplementary Table S1). This analysis revealed significant differences between piglets homozygous for Lr−0.23 and Lr−0.37 (p<0.05), and between piglets that were homozygous and heterozygous for Lr−0.23 (Lr−0.23 and Lr−0.23, −0.37; p<0.05). Taken together, these results indicate that the Lr−0.23 haplotype might be involved in, or a marker of, a relatively low birth weight in this population of Microminipigs.
We then determined the mean body weights of piglets in each haplotype group (n = 1,900) at postnatal day 50 (Figure 3). The mean body weight of piglets with the Lr−0.23 haplotype (3.14 kg) was significantly lower than that of piglets with the Lr−0.13 haplotype (3.46 kg, p<0.01). The mean weights of piglets at postnatal day 50 were examined in homozygous and/or heterozygous piglets with different SLA class II haplotypes (Supplementary Table S1). The mean weight of piglets with Lr−0.18, Lr−0.37 was significantly higher than that of piglets homozygous for Lr−0.23 and the heterozygotes Lr−0.11, 0.17, Lr−0.16, 0.37, Lr−0.18, 0.23, and Lr−0.23, 0.37. Therefore, consistent with the comparative birth weight results, piglets with the Lr−0.23 haplotype had relatively low body weights at 50 days of age. In contrast, piglets with Lr−0.13 had relatively heavier body weights at 50 days of age. Moreover, our data indicate that piglets with the Lr−0.18, 0.37 heterozygous haplotype tended to be heavier than other piglets (Supplementary Table S1).

Body weights of piglets with each swine leukocyte antigen (SLA) class II haplotype at 50 days of age. Columns and bars indicate means and standard error, respectively. The number of haplotypes was counted as two in homozygous individuals. The data from Lr−0.7 were excluded from calculations due to the small number of piglets with this haplotype. White and black columns represent the lower and higher mean values of Lr−0.23 and Lr−0.13, respectively, with significant differences among haplotypes. Gray columns represent mean values that did not differ significantly from those of other haplotypes.
The DGs of piglets of each SLA class II haplotype were mea sured between birth and postnatal day 50 (Figure 4). No significant differences in DG were observed among the eight haplotype groups. However, piglets with heterozygous haplotypes containing Lr−0.23, in combination with Lr−0.16, Lr−0.17, Lr−0.18, and Lr−0.37, as well as Lr−0.11, 0.18 had significantly lower DGs than those of piglets with Lr−0.18, 0.37. Furthermore, a significantly lower DG was observed in heterozygous piglets with the Lr−0.11, 0.18 haplotypes than in those with the Lr−0.18, 0.37 haplotypes (p<0.05).

Effect of swine leukocyte antigen (SLA) class II haplotypes on daily gains between the day of birth and 50 days of age in Microminipigs. Columns and bars indicate means and standard error, respectively. The number of haplotypes was counted as two in homozygous individuals. The data from Lr−0.7 were excluded from calculations due to the small number of piglets with this haplotype. No significant difference was observed in daily gains between the haplotypes.
DISCUSSION
Previously, we identified eight distinct SLA class II haplotypes in the Microminipig population [13]. In contrast, four or fewer SLA class II haplotypes have been identified in most other miniature pig breeds. For instance, four, three, and two class II haplotypes have been reported in Yucatan [15], NIH [16], and Clawn minipigs [17], respectively. Theoretically, it is possible to establish eight SLA class II homozygous Microminipig lines. These homozygous lines would be useful for immunological research, including organ transplantation studies, examinations of associations between SLA haplotypes and disease resistance, or studies of production traits.
Previous studies of the association between SLA and produc tive and reproductive traits have indicated that the SLA complex influences swine production and immune traits (reviewed in [18]). Recently, the investigation of a putative quantitative trait locus (QTL) affecting growth and fatness excluded SLA as a candidate region, suggesting that SLA markers are not useful for production traits [12]. However, in a Meishan/Large White composite population, Wei et al. found a QTL affecting growth traits located near the SLA region, suggesting the possibility of applying SLA allele diversity as a marker-assisted strategy [11]. In fact, our results suggested that Lr−0.23 is a useful genetic marker in the breeding process for selecting animals with relatively small body sizes in the Microminipig herd, but we have not performed QTL analyses using genetic markers in the SLA region in this herd and other pig populations. In addition, SLA haplotypes, assigned by serology, influence birth and weaning weights according to a study of 154 NIH miniature pigs [9]. Moreover, in Large White pigs, weaning weights were highest in pigs with a SLA class I haplotypes 13, 1, and 3 [19].
A characteristic feature of Microminipigs is their small body size. Therefore, it is important to clarify the relationship between SLA class II haplotypes and factors that contribute to small body size, including body weight and growth rate. In this study, Microminipigs with the Lr−0.23 haplotype had relatively low body weights at birth and at postnatal day 50 compared to those of other haplotypes. Furthermore, piglets with heterozygous haplotypes containing Lr−0.23 and Lr−0.16, Lr−0.17, Lr−0.18, or Lr−0.37, had significantly lower DGs than those of heterozygous piglets with the Lr−0.18, 0.37 haplotype. However, no significant differences in mean DG were observed among the eight SLA class II haplotypes. These results suggest that the Lr−0.23 haplotype in Microminipigs is associated with a low body weight. The effects of SLA class II haplotypes on body weight and the growth rate appear to be minor. In particular, there was little effect on growth rate between days 0 and 50 in the piglets. Nevertheless, SLA haplotypes may assist in the selection and breeding of Microminipigs with smaller body sizes. Lr−0.23 was the most frequent haplotype observed and accounted for 36.9% of the SLA class II haplotypes detected in the 950 Microminipigs analyzed. It is likely that piglets with the Lr−0.23 haplotype were preferentially selected owing to their relatively low body weights during the establishment of the Microminipig population. Piglets with the Lr−0.23 haplotype are healthy with normal growth and body shapes. Therefore, this SLA class II haplotype may be a suitable marker for the selective breeding of Microminipigs with low body weights. Microminipigs with small body sizes have advantages in the laboratory setting with respect to handling, treatment, environmental load, and the costs of breeding and examinations. However, further analyses of other breeds are required to confirm that the Lr−0.23 haplotype determines minipig body weight and growth rate and that the relationship is not restricted to Microminipigs.
Supplementary Information
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
