Antioxidant potential of buffalo and cow milk Cheddar cheeses to tackle human colon adenocarcinoma (Caco-2) cells
Article information
Abstract
Objective
The aim of present study was to assess the anti-oxidant potential of water-soluble peptides (WSPs) extract derived from buffalo and cow milk Cheddar cheeses at different stages of ripening.
Methods
The antioxidant potential of WSPs extract was assessed through 2,2’-azinobis-3-ethylbenzothiazoline-6sulfonic acid (ABTS)-radical scavenging activity. In addition, impact of WSPs extract on cell viability and production of reactive oxygen species (ROS) in human colon adenocarcinoma Caco-2 (tert-butylhydroperoxide-induced) cell lines was also evaluated.
Results
The ABTS-radical scavenging activity increased progressively with ripening period and dose-dependently in both cheeses. However, peptide extract from buffalo milk Cheddar cheese demonstrated relatively higher activity due to higher contents of water-soluble nitrogen. Intracellular ROS production in Caco-2 cells decreased significantly (p<0.05) till 150th day of cheese ripening and remained constant thereafter. Additionally, dose-dependent response of WSPs extract on antioxidant activity was noticed in the Caco-2 cell line.
Conclusion
On the basis of current in vitro study, the Cheddar cheese WSPs extract can protect intestinal epithelium against oxidative stress due to their antioxidant activity.
INTRODUCTION
Oxidative stress has been implicated in the development of various chronic ailments such as cardiovascular diseases, arthritis, inflammation and cancer [1]. It also affects the gastrointestinal system and creates other degenerative diseases. Reactive oxygen species (ROS) impart undesirable effects on sensory properties of foods, producing rancid odors and flavors which can reduce their shelf life, nutritional quality and safety [2]. Synthetic antioxidants have been added in food products to minimize the oxidative deterioration, although these may also impose potential risks to human health [3]. In this context, natural antioxidants with chemotherapeutic and preservation properties have greater demand in current market. The protein hydrolysates and bioactive peptides isolated from fermented dairy products could be utilized in functional foods to reduce the risk of various chronic ailments associated with oxidative stress [4].
Milk is preserved through fermentation into different products especially a vari ety of cheeses. Cheddar cheese shows a diverse proteolytic system during ripening through the action of proteinases and peptidases from milk indigenous enzymes, coagulants, starter and non-starter lactic acid bacteria and adjuncts [5]. Proteolysis results in the formation of large-(water-insoluble) and intermediate-sized (water-soluble) peptides which are further hydrolysed to produce small peptides and amino acids. Crude extract of water-soluble peptides (WSPs) may synergistically act to perform several physiological functions as antihypertensive, antioxidative, antiinflammatory, anticancer, antibacterial, antithrombotic, and cholesterol lowering properties [6]. Production and functional role of peptides is related with the proteolysis in ripened cheeses. Therefore, cheese ripening could be an important factor affecting the bioactivities of peptides.
Cellular anti-oxidant assays are biologically more represen tative methods demonstrating a better prediction of antioxidant behavior in biological systems. Thus direct evaluation of intracellular ROS is a good indicator of oxidative damage to living cells. Only few studies have been conducted for evaluating the antioxidative potential of protein hydrolysates or isolated peptides using cell cultures [7–9] and animal models. However, to date, no information about the antioxidant effect of WSPs extract derived from Cheddar cheese against cellular oxidative stress has been reported. Therefore, current study aimed to assess the in vitro antioxidant capacity of WSPs extract through 2,2′-azinobis-3- ethylbenzothiazoline-6sulfonic acid (ABTS)-radical scavenging potential and using human colon adenocarcinoma Caco-2 cell line model system.
MATERIALS AND METHODS
Manufacture of Cheddar cheese
Cheddar cheese was manufactured (triplicate batches for each) from buffalo and cow milk [10]. The whole pasteurized milk was first standardized to a casein to fat ratio of 0.7. After taking out cheese from moulds, it was packed in oxygen barrier bags, vacuum sealed and stored for ripening at 8°C for 6-months.
Preparation of water-soluble peptides extract of Cheddar cheeses
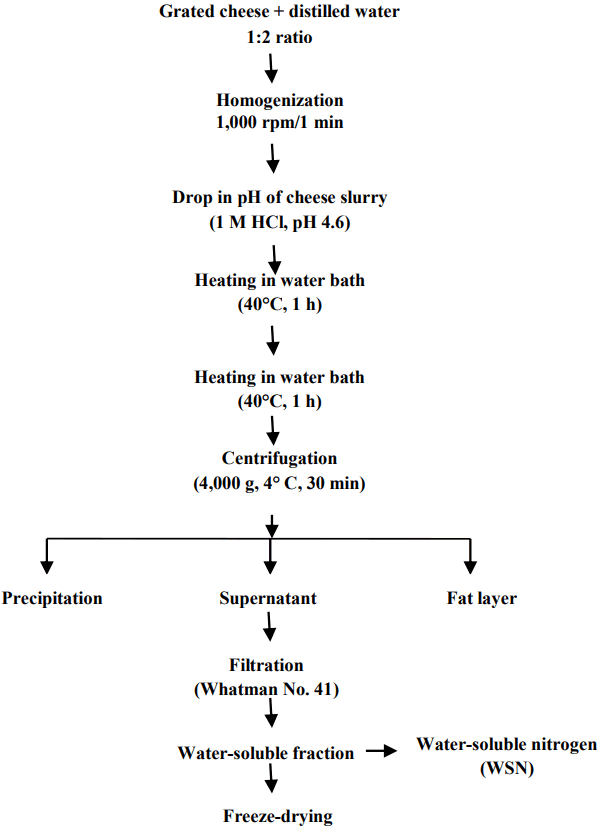
The WSPs extracts were prepared [11] at different stages of cheese ripening, freeze-dried (CHRIST, ALPHA 1–4 LD plus, Germany) and stored at −20°C for further analysis (Figure 1). Water-soluble nitrogen (WSN) contents of these extracts were calculated during cheese ripening at the interval of 2-months.
Antioxidant activity of water-soluble peptide extract
Scavenging activity of ABTS-radical
Antioxidant potential of WSPs extract was measured by ABTS-radical scavenging activity assay [12]. The radical cation ABTS· was produced by reacting 5 mL of ABTS (Sigma-Aldrich, St. Louis, MO, USA) stock solution (7 mM) with 88 μL of K2SO4 solution (2.5 mM) and mixture allowed to stand in dark at room temperature for 12 to 16 h before use. For the assay, the ABTS+· solution was diluted with 5 mM phosphate buffered saline pH 7.4 (PBS) to an absorbance of 0.70± 0.02 at 734 nm. A 20 μL of WSPs extract sample (5, 10, 15 mg/mL) was mixed with diluted ABTS·+ solution (1 mL) and incubated at 30°C for 6 min. The absorbance was measured at 734 nm using spectrophotometer (Elx800TM absorbance micro plate reader, BioTek instrument, Winooski, VT, USA) and plotted as a function of reference antioxidant (Trolox, Sigma-Aldrich, USA). Similarly, distilled water (20 μL) was used for the blank. Radical-scavenging activity was calculated by equation;
Cell viability assay
The Caco-2 cell lines (ATCC, Manassas, VA, USA) were maintained in Dulbecco’s modification of Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 U/mL), streptomycin (0.1 mg/mL) and non-essential amino acid (1%) solution at 37°C in a humidified atmosphere (95% air, 5% CO2). Cells were kept sub-confluent and media were changed after every 3 to 4 days. All cells used in experiments were between 4 and 25 passages.
Cell viability was measured using the 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [13] which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenases. Cells were seeded in 96 well plates following 24 h incubation at 37°C in a humidified atmosphere (95% air and 5% CO2). At 24 h, cells were treated with different concentrations (100, 200, and 300 μg/mL) of WSPs extract of media (200 μL). The formazan dye was solublized by adding 100 μL of dimethyl sulfoxide (Fisher scientific CHEMTREC, Fair Lawn, NJ, USA) to each well. The absorbance was measured at 570 nm by using a plate reader (Elx800TM, BioTek Instrument, USA).
Cellular ROS determination by 2′,7′-dichlorofluorescin-diacetate assay
The intracellular ROS formation in human colon adenocarcinoma Caco-2 cell model was quantified [14] with some modifications using DCFH (Sigma-Aldrich, USA) as fluorescent probe. Caco-2 cells (200 μL) were seeded in black fluorescence microtiter 96-well plates (1.5×104 cells per well) and incubated (37°C, 24 h) in a humidified incubator (5% CO2, 95% air). Then, culture medium was removed and cells were pretreated with 200 μL of 100, 200, and 300 μg/mL and incubated for 24 h. Culture medium was removed again and washed with PBS (pH 7.4). Subsequently, 10 μM 2′,7′-dichlorofluorescin-diacetate (DCFH-DA) (100 μL) in PBS (pH 7.4) was added and incubated in dark for 30 min. Afterward, 100 μL of 0.3 mM tert-butyl hydroperoxide (t-BHP) in PBS (pH 7.4) was added and incubated for 1 h. DCFH was oxidized by ROS to form a fluorescent product, 2′, 7′-dichlorofluorescin (DCF), which was monitored at the excitation wavelength (Ex) of 485 nm and the emission wavelength (Em) of 528 nm using a fluorescence microplate reader (Biotek microplate reader, GEN [GEN5.19] software, Winooski, VT, USA). The fluorescence intensity is related to the amount of oxidative damage, and a reduction in fluorescent intensity is proportional to the antioxidant protection provided by the sample.
Statistical analysis
The data was analyzed statistically by analysis of variance using Minitab statistical package and Tukey’s test was used for multiple comparisons (∝ = 0.05) between means. Results were expressed as mean values±standard error of triplicate analyses. Simple linear (Pearson’s correlation) correlation was used to determine a relationship between the mean values of WSN and antioxidant activities.
RESULTS AND DISCUSSION
Anti-oxidant activities
ABTS-radical scavenging activity
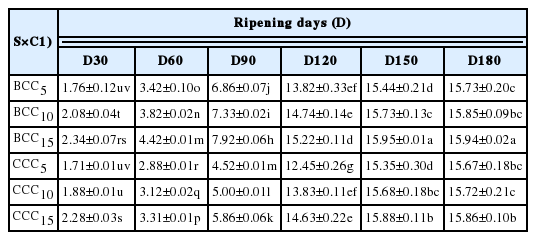
ABTS being water-soluble could easily diffuse to target peptides of aqueous nature. Therefore, antioxidant compounds of both hydrophilic and lipophilic character can be measured by ABTS-radical scavenging assay [15]. In present study, antioxidant activity of WSPs extract was increased throughout ripening period, demonstrating greatest inhibition values 15.95% (BCC) and 15.88% (CCC) at 150th day. There was drastic increase in activity after 90 days of ripening till the end of cheese maturation. Non-significant differences were observed for the activity at days 150 and 180 ripening in both cheeses (Table 1). Moreover, significant (p<0.05) dose-dependent response of WSPs extract was evidenced for the activity, indicating higher radical quenching potential at 15 mg/mL concentration till 150th day of cheese ripening.
The scavenging potential of Cheddar cheese water-soluble ex tracts is dependent on ripening and it was found to increase with ripening stages [16] and concentration of peptides [17]. Similar to current finding, the dose-dependent effect of yak casein hydrolysate on scavenging activity was observed [18]. The structure, configuration, size, and amino acid sequence of peptides may also affect bioactivity [19]. Peptides comprised of glutamine, lysine, methionine, tyrosine, histidine, cysteine and proline possess strong antioxidant activity. The proline residues of bioactive peptides were reported in water-soluble extract of Roquefort cheese. Likewise, leucine and valine released from β-CN (211 to 220) and αS1-CN (121 to 130) are also capable of quenching the radicals, respectively [20].
Effect of WSPs extract on cell viability
Cell culture models are used to evaluate cytotoxicity of antioxidative compounds at concentrations calculated to exert the desired bioactivity in the body and to study the potential to inhibit intracellular oxidation. The treatments of WSPs extract evoked no changes in cell viability (data not shown) of Caco-2 cells, representing that the concentrations selected for study (100 to 300 μg/mL) did not damage cell integrity during incubation period.
Intracellular ROS production in Caco-2 cells
Human adenocarcinoma colon (Caco-2) cell monolayers have been extensively studied in vitro model for studying intestinal permeability of bioactive components due to their similarity to intestinal endothelium cells. These cells form tight junctions to attain morphological and functional characteristics of enterocytes. Smaller peptides may be absorbed intact across the brush border membrane [21].
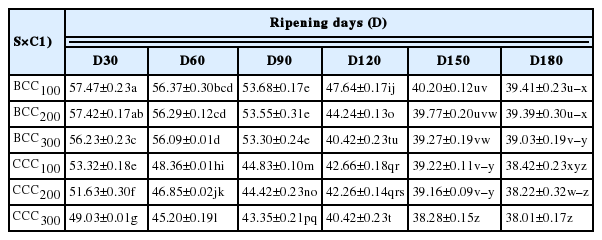
Results of current study depicted that ROS production in Caco-2 cells decreased significantly (p<0.05) upon treatment with WSPs extract obtained at different stages of Cheddar cheese ripening. However, the WSPs fraction collected at 150th and 180th day of ripening showed maximum reduction in ROS production in cell line. At the highest dose (300 μg/mL), BCC and CCC demonstrated 17.4% and 11.02% drop in ROS levels in t-BHP- stressed Caco-2 cells from 30 day to 180 day of cheese ripening respectively, (Table 2).
In the present study, quantification of intracellular ROS pro duction was conducted using DCFH as fluorescent probe. Upon cellular uptake, DCFH-DA probe is hydrolyzed to DCFH by intracellular esterase and oxidized to highly fluorescent DCF in the presence of ROS. The extent of oxidation can therefore be measured by fluorescence intensity which is a good marker of overall oxidative stress in cells [22]. A simulated gastrointestinal digestion of phosvitin phosphopeptide reduced proinflammatory interleukin secretion in Caco-2 cells and highlighted the potential of the peptide to prevent oxidative stress and promote gut health [23]. These results suggest that WSPs extract can protect cells from oxidative damage by ROS, thus it may be developed into a potential bio-molecular candidate to inhibit ROS formation.
Production of ROS is considered cytotoxic and associated with various degenerative ailments. At gastrointestinal level, increased concentrations of ROS have been related to the development of intestinal pathologies [24]. Hence, there is an interest in assessing the potential of food antioxidants to protect intestinal epithelium against oxidative stress. The caseinophosphopeptides have demonstrated an ability to preserve viability and protect against oxidative damage at intestinal (Caco-2 cells lines) level [11]. Similarly, the casein and whey protein hydrolyzates were previously reported to have protective effects in H2O2-induced hepatic (HepG2) and neuronal (PC-12) cells, respectively [25,26]. Multifunctional character of peptides have the potential to maintain ROS concentrations at low levels as evidenced by in vitro antioxidant studies [27]. These are also considered to be safe and healthy compounds with low molecular weight, high activity and easy absorption. Furthermore, peptides can act synergistically with non-peptide antioxidant enhancing their protective effect [9].
Relationship of Cheddar cheese water-soluble nitrogen contents with antioxidant activities
The WSN contents increased steadily during cheese ripening, though it was relatively higher (26.34%) in cheese made with buffalo milk (Table 3) which can be correlated with higher content of casein and its fractions, particularly β-casein, in buffalo milk [28] compared with cows’ milk. Progressive increase in the level of WSN in cheese was also demonstrated by previous research studies [29,30].
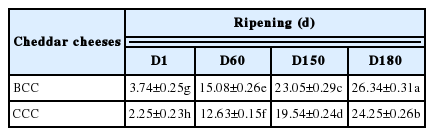
Water-soluble nitrogen (WSN) contents of buffalo milk Cheddar cheese (BCC) and cow milk Cheddar cheese (CCC) at different stages of ripening
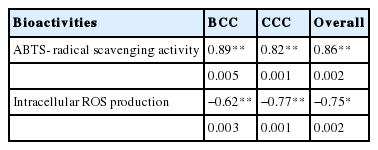
It was established that degree of ripening and the rate of soluble peptides production in cheeses are also related to the anti-oxidant activity [17,31]. Highest radical quenching action in BCC could also be evidenced with higher contents of WSN reported in the present investigation. Correlation between WSN and ABTS-scavenging activity was observed from Pecorino Toscano (r = 0.98), Pecorino Sardo (r = 0.93), and Cerrillano (r = 0.93) cheeses [10]. However, the ripening index designated by WSN contents of cheeses showed negative relationship with cellular antioxidant activity (Table 4) as the increase in activity was noticed till the 150th day of ripening but decreased again at 180th ripening day. The observed antioxidant activities of Cheddar cheese water-soluble extract advocate that they can be used as food preservation, functional and nutraceutical ingredient. The water-soluble crude extracts of cheeses comprised of major peptides formed during ripening may exert several physiological roles including antioxidant action. Therefore, it would be economically more expedient and feasible to use crude or semi-purified peptide extract in food matrices.
CONCLUSION
The results suggested that WSPs extract derived from Cheddar cheese can scavenge free radicals and inhibit radical mediated oxidation in Caco-2 cells. It provides great promise that these peptidic extracts may significantly contribute to preserve the integrity of intestinal tissues against oxidative damage. However, animal studies and human clinical trials should be needed to conduct to confirm bioavailability and the desired biological function in vivo.
ACKNOWLEDGMENTS
Authors acknowledged the Higher Education Commission (HEC) Pakistan for financial support and University of Massachusetts, Amherst, MA, USA for ftechnical support.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.