Effects of dietary supplementation with detoxified Rhus verniciflua sap on egg production, yolk lipid and intestinal microflora in laying hens
Article information
Abstract
Objective
This study was conducted to investigate the effects of dietary detoxified Rhus verniciflua sap (RVS) on production performance, egg quality, lipid fractions of egg yolk, liver and serum, and the profile of cecal microflora in laying hens.
Methods
Two hundred 52-week-old Hy-Line Brown layers were randomly divided into 4 groups with 5 replicates per group (2 hens per cage, 5 cages per replicate) and were provided with one of 4 experimental diets containing 0%, 0.05%, 0.1%, or 0.2% RVS, for 6 weeks. Due to unequal intervals of RVS doses, the interactive matrix language procedure of the SAS program was used to correct the contrast coefficients of orthogonal polynomials.
Results
There were no differences in feed intake and egg weight among the groups. Egg production increased (linearly and quadratically, p<0.05) with increasing levels of RVS. Eggshell thickness increased (linear, p<0.05) as the level of RVS in diets increased. The levels of blood cholesterol and activities of glutamic-oxaloacetic transaminase and glutamic-pyruvic transaminase were not altered by dietary treatments. Increasing level of RVS increased (linear, p<0.05) the populations of cecal lactic acid bacteria. The content of yolk cholesterol decreased (linear, p< 0.05) with increasing levels of dietary RVS, although there were no significant differences in each lipid fraction of the liver.
Conclusion
This study indicates that dietary RVS could improve laying performance and eggshell quality, and affect cecal lactic acid bacteria in a dose-dependent manner.
INTRODUCTION
Bioactive substances derived from plants can play important roles in improving both productive performance and immune status of poultry [1]. Beneficial effects of plant substances in poultry include the stimulation of feed intake, the improvement of growth and laying performance, the activation of immune system and antibacterial and antioxidant actions [2,3]. Rhus verniciflua (RV) is a plant species in the Anacardiaceous family and it grows widely in Northeast Asian countries [4]. Traditionally, it has been used for the protection of antiquities and for treating gastritis and atherosclerosis as an oriental medicine [5]. The Rhus verniciflua sap (RVS) is composed of urushiol (60% to 65%), gummy substrates (4% to 8%), glycoprotein (2% to 5%), laccase (1%) and water (20% to 25%) [6]. Among them, urushiol is an oily organic component and exerts several beneficial effects such as antimicrobial [7], and antioxidant [8] activities. Due to the afore-mentioned effects [7,8], RV and urushiol were tested as the feed supplements to enhance health and/or productivity in pigs [9,10] and broiler chickens [11]. However, it is reported that urushiol might have toxic effects as it is the main antigen of Rhus and exhibits strong antigenicity in animals [12]. Thus it is recommended to be detoxified before practical use, e.g., by employing polymerization process via simple heating [12]. Unfortunately, the RV products used in animal feeding trials [10,11] were not detoxified albeit that there are no studies reporting detrimental effects of intact (i.e., un-detoxified) RVS on animal performance and general health of livestock. In addition, information on the dietary effects of detoxified RVS on laying performance and physiological responses in commercial layers is very limited, which prompted us to set up the current experiment to evaluate the dietary effect of detoxified RVS on egg production, egg quality, lipid fraction of liver and egg yolk in laying hens.
MATERIALS AND METHODS
Test substrate
The detoxified RVS was provided by Celltech Co. Ltd. The raw sap was harvested from Rhus verniciflua trees that were more than 12 years old. It was mixed with ethanol (1:1, v/v) and stirred evenly. The stirred raw RVS-ethanol mixture was centrifuged at 10,000×g for 30 min for 4°C. The resulting supernatant was vacuum-dried to remove ethanol and water, and then mixed with cereal mixture (1:9, w/w). The final mixture containing the extract was heated in an oven at 90°C for 4 d and ground and homogenized thoroughly to obtain detoxified RVS [12].
Animals, diets and management
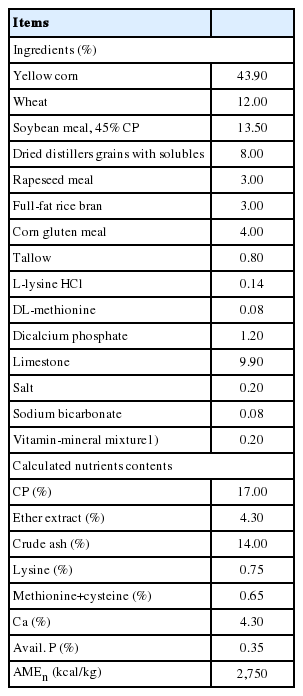
Two hundred 52-wk-old Hy-Line Variety Brown hens were randomly divided into 4 treatment groups. Each treatment had 5 replicates with 5 cages each and 2 hens per cage (35×40 = width× length; 700 cm2/hen) so that each treatment had 50 hens (10 hens/replicate, 50 hens/treatment). The layers were fed one of the four experimental diets with 0%, 0.05%, 0.1%, or 0.2% detoxified RVS, respectively. All diets were formulated to meet or exceed the nutrient requirements of NRC [13] for Brown laying hens, as shown in Table 1. The experiment lasted 6 weeks and diets and water were provided on an ad libitum basis. A room temperature of 20°C±3°C and a photoperiod of 16/8 h light/dark cycle were maintained throughout the experimental period. Diets were freshly added daily and feed intake was recorded weekly. The protocol for the experiment was approved (KU15186) by the Institutional Animal Care and Use Committee at Konkuk University.
Egg production and qualities
The number and weight of eggs laid were recorded daily. The abnormal eggs (i.e., soft-shell, shell-less, or broken eggs) were excluded for egg weight measurement. Egg mass was calculated as hen-day egg production multiplied by the average egg weight. The percentages of cracked eggs were calculated by replicate (number of soft-shell and broken eggs/number of eggs produced×100). At 2, 4, and 6 weeks of experiment, five eggs from each replicate were collected, weighed individually and stored overnight at room temperature for subsequent measurements.
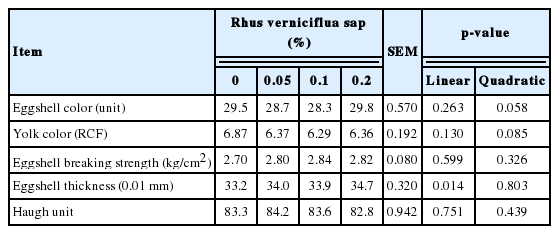
The breaking strength of intact eggs was measured with an eggshell strength tester (FHK, Fujihara Ltd., Tokyo, Japan). Eggshell thickness without shell membrane was tested by micrometer (Digimatic micrometer, Series 293–330, Mitutoyo, Japan). Eggshell color and albumin height were measured by using Egg multi-tester (QCM+ Technical Services and Supplies Ltd., York, England). Haugh unit, along with albumen height and egg weight, was calculated as previously described [14]. Egg yolk color was measured with Roche yolk color fan (Hoffman-La Roche Ltd., Basel, Switzerland).
Sampling and measurements
At the end of the experimental period, one bird per replicate (5 birds/treatment) were selected and weighed individually. The blood samples were taken from the jugular vein in vacutainer tubes. Serum was obtained by gentle centrifugation (2,000×g) for 15 min and stored at −60°C until analysis. The levels of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), and total cholesterol were measured according to the colorimetric method using biochemical analyzer (Hitachi modular system, Hitachi Ltd., Tokyo, Japan). At necropsy, liver and abdominal fat were immediately removed and weighed. Then, liver was frozen for subsequent determination of the contents of each lipid fraction.
The total lipids were extracted from egg yolk and liver using chloroform/methanol (2:1, v/v) as described by Folch et al [15]. The contents of each lipid fraction in the liver and egg yolk were separated by thin layer chromatography on silica gel chromatorods using hexane:diethylether:formic acid (85:15:0.1, v/v) as developing solvents, and quantified by IATRO SCAN (TH-10 TLC/FID analyzer, Iatron Laboratory. Inc, Tokyo, Japan), with hydrogen as gas flow [14].
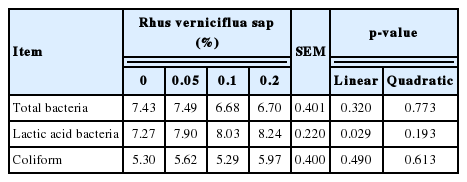
The cecal content was aseptically sampled for microbial test. The cecal digesta homogenates in sterile, ice-cold PBS were serially diluted from 10−1 to 10−7. Dilutions were plated on selective agar for enumeration of target bacterial strains. Total bacteria, coliforms, and Lactobacillus spp. were enumerated using total plate agar, MacConkey agar, and MRS agar, respectively. Each plate was incubated at 37°C, for 24 to 72 h and colonies were then counted. Results obtained were presented as base-10 logarithm colony-forming units per gram of cecal digesta [16].
Statistical analysis
Obtained data were analyzed using the general linear model procedure of SAS software [17]. Orthogonal polynomial contrast was used to determine the linear and quadratic effects of dietary RVS. The interactive matrix language procedure of the SAS program was used to correct the contrast coefficients of orthogonal polynomial due to unequal intervals of RVS doses. Results were considered significant if the p-values were less than or equal to 0.05.
RESULTS
Hen-day egg production increased (linear and quadratic, p<0.05) with increasing dietary RVS levels (Table 2). There were no significant differences in feed intake and egg weight among treatments. The graded levels of dietary RVS increased (linear and quadratic, p<0.05) egg mass. The relative weights of liver and abdominal fat were not affected by the level of dietary RVS. Eggshell thickness was improved (linear, p<0.05) with increasing dietary RVS (Table 3). However, eggshell color, yolk color, eggshell strength, and Haugh unit were not influenced by dietary treatments. The incidence of cracked eggs was negligible across the treatments (data not shown). Both GOT and GPT activities, and total cholesterol in serum samples were not altered by dietary treatments (Table 4). Dietary RVS failed to affect the contents of cholesterol, triacylglycerol and phospholipid in liver. However, the cholesterol contents in the egg yolks decreased (linear, p<0.05) with increasing concentration of dietary RVS. Finally, the supplemental RVS increased (linear, p<0.05) the population of cecal lactic acid bacteria, but did not affect the numbers of total and coliform bacteria (Table 5).

Effects of dietary detoxified Rhus verniciflua sap on egg performance and relative organ weights in laying hens

Effect of dietary detoxified Rhus verniciflua sap on blood profiles and lipid fractions of liver and egg yolk in laying hens
DISCUSSION
The RV extract is used to treat gastritis and atherosclerosis as oriental medicine [5], making it potentially useful for domestic animals. It has been reported that dietary RV extract increased fat digestibility in broiler chickens [11] and improved the oxidative stability of pork meats [10] without affecting growth performance. In this study, we evaluated the effects of graded levels of RVS on laying performance, egg quality, lipid metabolism and cecal microflora in laying hens. It was noted that dietary RVS supplementation increased laying performance and egg qualities compared with the control group. In line with our study, Kang et al [18] reported that egg production and egg mass were significantly increased in laying hens that consumed RV extracts added to drinking water. The latter study also found that RV extracts increased digestibility of crude protein and crude ash [18]. Thus, an increase in laying performance and egg quality seen in our study and others [18] may in part relate to RVS-mediated enhancement in utilization of nutrients.
The levels of GOT, GPT, and total cholesterol were not affected by dietary treatments in this study (Table 4). Measurement of blood GOT and GPT activities, indicative of liver and tissue damages in avian, is a valuable tool to determine a safe application for new feedstuffs and feed additives [19]. Urushiol is an antigen component in RV products [12] and it can cause an allergic skin rash on contact, known as urushiol-induced contact dermatitis [20]. Therefore, it would be considered beneficial to detoxify RV products containing urushiol [12] although any negative effects of the RV on productivity and physiological responses in livestock have not been reported. In any event, dietary detoxified RVS used in this study did not affect GOT and GPT activities, indicating its safe use as a feed additive for laying hens.
A cholesterol lowering effect of Rhus species in experimental animals has been reported. For example, the dietary supplementation of glycoprotein derived from RV stokes lowered the levels of triacylglycerol and low density lipoprotein-cholesterol in mice [21]. Golzadeh et al [22] found that dietary Rhus coriaria reduced the level of very low density lipoprotein-cholesterol in broiler chicks, which can be related to decreased activity of 3 hydroxy-3 methylglutaryl CoA reductase, a rate-limiting enzyme in the synthesis of cholesterol. In addition, the synthesis of hepatic bile acids was significantly increased in RV extract-fed mice [23]. To our knowledge, there are little data available concerning the effect of dietary RV or RV extracts on the content of yolk cholesterol in commercial laying hens. In this study, yolk cholesterol, but not blood and liver cholesterol, was significantly decreased by dietary RVS in a linear manner (Table 4). It is well-known that egg cholesterol is synthesized in the liver, secreted into the blood as very low-density lipoprotein and deposited to yolk via receptor-mediated endocytosis [24]. Thus, a reduction in yolk cholesterol by dietary RVS as seen in this study may be the consequences of altered cholesterol metabolism (i.e., synthesis, degradation and tissue redistribution) and/or endogenous lipoproteins (i.e., production and secretion). It is considered important that the reduction in egg cholesterol content was not accompanied by lower egg production.
The supplemental RVS increased (linear, p<0.05) the population of cecal lactic acid bacteria although there were no significant differences in the number of total and coliform bacteria (Table 5). Our finding is consistent with that of Kang et al [18], who reported that the laying hens given RV extract-added drinking water had similar numbers of cecal Escherichia coli and salmonella. The observation on RVS-induced increase in cecal lactic acid bacteria seems promising as lactobacillus can produce lactic acid to maintain a low pH or inhibit pathogen overgrowth in chickens [25]. Nonetheless, the latter interpretation should be cautious since the conventional culture-dependent technique that we used in this study is time-consuming and prone to misinterpretation [26] for microbial ecology analysis. Thus, incorporation of molecular-based approach [27] will clarify the RVS-induced shift in gut microbiota of laying hens.
CONCLUSION
It is concluded that dietary RVS improved egg production and eggshell quality in a linear manner in laying hens. The detoxified RVS increased the population of cecal lactic acid bacteria and lowered the cholesterol contents of the egg yolk.
ACKNOWLEDGMENTS
This paper was supported by Konkuk University in 2016. The authors thank Dr. Oh Sung-Taek in the Agricultural Research Service of the United States Department of Agriculture for proof reading the manuscript.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.