Physico-chemical properties of late-incubation egg amniotic fluid and a potential in ovo feed supplement
Article information
Abstract
Objective
This study explored the physico-chemical properties of late-incubation egg amniotic fluid and a potential in ovo feed (IOF) supplement.
Methods
Amniotic fluid was collected from broiler breeders (Ross 308, 51 weeks and Cobb 500, 35 weeks) on day 17 after incubation. A mixture of high-quality soy protein supplement – Hamlet Protein AviStart (HPA) was serially diluted in MilliQ water to obtain solutions ranging from 150 to 9.375 mg/mL. The mixtures were heat-treated (0, 30, 60 minutes) in a waterbath (80°C) and then centrifuged to obtain supernatants. The amniotic fluid and HPA supernatants were analysed for their physico-chemical properties.
Results
Only viscosity and K+ were significantly (p<0.05) different in both strains. Of all essential amino acids, leucine and lysine were in the highest concentration in both strains. The osmolality, viscosity and pCO2 of the supernatants decreased (p<0.05) with decreasing HPA concentration. Heat treatment significantly (p<0.05) affected osmolality, pH, and pCO2, of the supernatants. The interactions between HPA concentration and heat treatment were significant with regards to osmolality (p<0.01), pH (p<0.01), pCO2 (p<0.05), glucose (p<0.05), lactate (p<0.01) and acid-base status (p<0.01) of HPA solutions. The Ca2+, K+, glucose, and lactate increased with increasing concentration of HPA solution. The protein content of HPA solutions decreased (p<0.05) with reduced HPA solution concentrations. The supernatant from 150 mg/mL HPA solution was richest in glutamic acid, aspartic acid, arginine and lysine. Amino acids concentrations were reduced (p<0.05) with each serial dilution but increased with longer heating.
Conclusion
The values obtained in the primary solution (highest concentration) are close to the profiles of high-protein ingredients. This supplement, as a solution, hence, may be suitable for use as an IOF supplement and should be tested for this potential.
INTRODUCTION
The amniotic fluid of broiler breeder egg plays a vital role in the development of the embryo during incubation [1]. While egg yolk, albumen, allantoic fluid [2], and also amniotic fluid from turkey [3] have been extensively studied for some of their properties and chemical compositions, there are scant literature reporting the physical properties and chemical composition of the amniotic fluid of eggs from broiler breeders during late-incubation stage.
A prominent early nutrition technique is in ovo feeding (IOF). This is the administration of nutritive supplements into the amniotic fluid during the embryo’s development, usually at late-incubation phase [3]. Several researchers have reported positive effects of a large variety of nutrients and supplements such as amino acids [4], carbohydrates [5], vitamins [6], nucleotides [7], L-carnitine [8], hormones [9], and electrolytes [10] in IOF. However, there have been certain concerns as well. For example, a review highlighted that while in ovo nutrient administration appears to have a significant effect on hatching weights, other responses such as final weights and feed conversion efficiency are not consistent [11]. While improving hatching weight, reduced hatchability with the use of glucose [12] and amino acids [13] for IOF have been reported. This may be an indication that the properties and chemical composition of particular supplements or nutrients may negatively affect growth and development [10]. This failure to obtain tangible benefits may be due to a lack of suitable nutritional products for administration. Therefore, understanding the physical and chemical properties of the amniotic fluid of chicken egg during late-incubation period may be useful in developing the most potent supplements for use in IOF that will not alter the consistency of the internal environment of chicken egg during incubation.
There is a need to match IOF supplements to the properties of the amniotic fluid of late-incubation egg, into which these supplements are usually delivered. Uni and Ferket [3], for example attempted to establish the optimal osmolality of saline (NaCl), one of its physical properties as an IOF supplement. The value (400 to 600 milimosmoles [mOsm]) arrived at may not be applicable to all IOF supplements and nutrients as each of them possesses specific physico-chemical properties. Hence, there are possible limitations on use of these nutrients and supplements due to lack of adequate knowledge on their physical (pH, osmolality, viscosity, etc) and chemical (electrolytes, metabolites, amino acid contents) properties. de Oliveira [14] advised that future IOF research should focus on identifying a protein or amino acid source that is more appropriate for IOF feeding formulae, and then evaluate the effect of IOF with different early feeding strategies. Research is therefore, needed to investigate, acquire such knowledge and improve the potential use of such IOF supplements or nutrients.
This experiment was set up to determine the physical properties and chemical composition of amniotic fluid from late-incubation eggs and of different concentrations of a high-quality processed protein supplement, with a view to determine the suitability of the later as a potential IOF supplement for broiler chicks.
MATERIALS AND METHODS
Egg collection and incubation
Two hundred and forty fertile eggs, 120 each, from 51-week old Ross 308 and 35-week old Cobb 500 broiler breeders were obtained from a breeder farm (Baida Farms, Tamworth, Australia). The broiler breeders were subjected to the different dietary and management conditions as recommended by the individual breeding companies, although both are on the same farm. The eggs were incubated (R-com MARU 1000, Auto Elex Co. Ltd., GyeongNam, Korea) at the Centre for Animal Research and Training (CART) of the University of New England, Armidale, Australia. Eggs were weighed to a standard deviation of ±0.05 and then placed in crates in 4 replicates of 30 eggs each. The weights of eggs on first day were recorded, incubated at 37.5°C and 55% relative humidity and turned at 3 hours interval until day 17 of incubation.
On day 17 of incubation, eggs were brought out from the incubator and weighed. With an 18 gauge×1½ inch hypodermic needle, a hole was drilled through the egg air sac from the broad end of the egg to locate the amniotic cavity after which the amniotic fluid was aspirated using a 21 gauge×1½ inch needle into a 5 mL syringe from the amniotic cavity. About 4.5 mL of amniotic fluid was collected from eggs and transferred into 2.5 mL Eppendorf tubes in duplicates and placed on ice. Collected amniotic fluid samples were stored at −20°C until samples were analysed.
Preparation and serial dilution of HPA
A high-quality processed soy protein product, HPA, with known nutrient composition was identified as the test material for IOF. The product was suspended in MilliQ (MQ) water and serially diluted from 150 to 9.375 mg/mL in centrifuge tubes. Each sample was replicated four times and vibromixed for 10 minutes. Samples were heated for 30 or 60 minutes at 80°C in a waterbath, or assessed at room temperature. Samples were then centrifuged at 3,200 rpm (Model – Beckmans Bench top Centrifuge, Allegra 6R, Indianapolis, IN, USA) for 10 minutes and the supernatants transferred to new tubes. The supernatants were collected and stored at −20°C until they were analysed after defrosting.
Measurement of physical properties
Frozen amniotic fluid and HPA solution samples were first defrosted before analysing them for osmolality. The osmolality of each sample was determined using Wescor vapour pressure osmometer (Model 5500, Wescor Inc., Logan, UT, USA). Eight μL of each sample was aspirated into a micropipette tip. Reading was taken after 80 seconds micro-processor measurement cycle of the osmometer and recorded in mmol/kg. The viscosity of the samples was measured using the Brookfield Digital Rheometer (Model DV III) and recorded in m/Pas as described by Kemps et al [15]. An average shear rate of 225 and 1,300 L/s, and 30 and 200 rpm was maintained on the viscosity measurements for the late-incubation egg amniotic fluid and HPA solution samples, respectively. The samples were analysed for pH using the pH meter (Model pH/Ion S220, Metter Toledo AG, Greifensee, Switzerland).
Gas, electrolyte and metabolite analyses
The gas, mineral electrolyte and carbohydrate metabolite analyses of the amniotic fluid from late-incubation eggs and the HPA solutions were done using a benchtop blood gas analyser (Model ABL800 Flex, Radiometer Medical ApS, Bronshoj, Denmark). The analyser was set to aspirate samples using a 195 μL syringe. Samples were analysed for partial pressure of carbon dioxide (pCO2), electrolytes (cK+, cNa+, cCa2+, cCl−) and metabolites (cGlu and cLac) values and acid-base status (cBase(Ecf))c.
Crude protein determination and amino acid analysis
To determine crude protein content of the samples, their nitrogen content was first determined and used as a factor for protein using the LECO TruSpec Carbon and Nitrogen Analyser (LECO Corporation, St. Joseph, MI, USA) and crude protein content of sample was calculated using N×5.75 for amniotic fluid and N× 6.25 for HPA solutions. Samples of amniotic fluid and the different concentrations of HPA solutions were analysed for amino acids using the high sensitivity amino acid quantification technique of Waters AccQTag Ultra chemistry on a Waters ACQUITY UPLC system. Sixteen amino acids were analysed, including histidine, serine, arginine, glycine, aspartic acid, glutamic acid, threonine, alanine, proline, lysine, tyrosine, methionine, valine, isoleucine, leucine, and phenylalanine.
Data analysis
Data collected from the experiment were analysed with Minitab 17 [16] using the general linear model or one-way analysis of variance where applicable. Means were separated to identify significant differences using the Fisher test at 95% level of confidence.
RESULTS
Physical properties of amniotic fluid from late-incubation
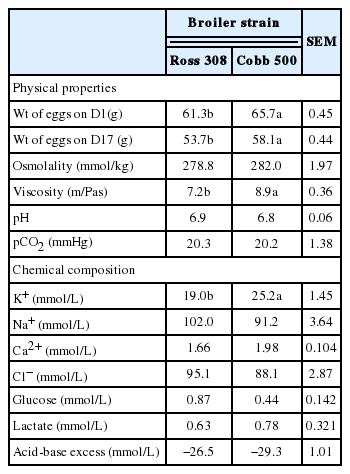
The physical properties of amniotic fluid from late-incubation (17 days) eggs are presented in Table 1. The eggs from Cobb 500 were heavier (p<05) than eggs from Ross 308. While eggs from Ross 308 lost 12.33% of the initial fresh mass (IFM) by 17 days after incubation, eggs from Cobb 500 lost 11.62% of the IFM after the same period (absolute egg weight loss). The viscosity of the amniotic fluid collected from Cobb 500 strain (8.92 m/Pas) eggs was significantly (p<0.05) higher than that of in fluid from Ross 308 (7.24 m/Pas). However, there were no significant (p>0.05) differences in the osmolality, pH, and pCO2 values of the late-incubation egg amniotic fluid from both strains.
Chemical composition of amniotic fluid from late-incubation
With the exception of K+, which was higher (p<0.05) in the late-incubation egg amniotic fluid of Cobb 500 eggs than that of Ross 308, the other mineral electrolytes and metabolites were similar (p>0.05) in the two strains (Table 1). However, marginal differences were observed across the mineral electrolytes and metabolites in both strains, with the percentage difference of about 65.65% in glucose content of the amniotic fluid of late-incubation from both broiler strains. Other differences in the mineral electrolyte and metabolite contents were about 20% or less. Also, sodium and chloride levels were in the highest concentration in the amniotic fluid of late-incubation eggs from both strains, while calcium was in the least concentration (1.66 and 1.98 mmol/L in Ross 308 and Cobb 500, respectively). While there was more glucose (0.87 mmol/L) than lactate (0.63 mmol/L) in amniotic fluid from late-incubation eggs from Ross 308, the reverse was observed in amniotic fluid from Cobb 500 (0.44 mmol/L glucose and 0.78 mmol/L lactate).
Protein content and amino acid profiles of amniotic fluid from late-incubation
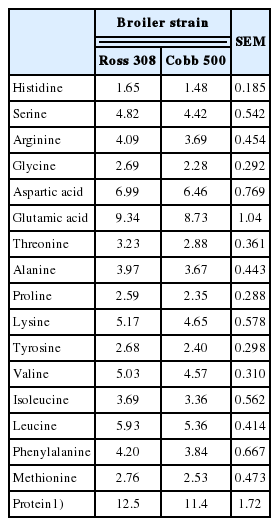
The results of the percentage protein and amino acid profiles of amniotic fluid from late-incubation eggs are presented in Table 2. The protein contents of amniotic fluid from both strains, based on sum of amino acids, were not significantly (p>0.05) different from each other. However, the value was marginally higher in the amniotic fluid of Ross 308 than Cobb 500. The amino acid contents of amniotic fluid of the eggs varied from 1.48 mg/g protein histidine to 9.34 mg/g protein glutamic acid. There were no significant (p>0.05) differences in the amino acid profile of amniotic fluid of the two broiler breeder strains. Glutamic acid was most abundant (9.34 and 8.73 mg/g protein in Ross 308 and Cobb 500, respectively), while histidine was the least abundant (1.65 and 1.48 mg/g protein in Ross 308 and Cobb 500, respectively). Of all the essential amino acids, leucine and lysine were in the highest concentration in both strains (5.93 and 5.17 mg/g protein in Ross 308, and 5.36 and 4.65 mg/g protein in Cobb 500, respectively). On the other hand, histidine was the lowest in concentration among the essential amino acids (1.65 and 1.48 mg/g protein in Ross 308 and Cobb 500, respectively).
Physical properties of different concentrations of HPA solutions
The osmolality, viscosity and pCO2, all decreased (p<0.05) with reduction in HPA concentration (Table 3). The HPA solution at the highest concentration of 150 mg/mL had the highest (p<0.05) osmolality (217.78 mmol/kg), viscosity (1.66 m/Pas), and pCO2 (8.08 mmHg), while the HPA solution of 9.375 mg/mL had the lowest (p<0.05) osmolality (63.11 mmol/kg), viscosity (1.10 m/Pas), pCO2 (5.30 mmHg). A reverse trend was observed in pH values with the lowest concentration (9.375 mg/mL) having the highest pH (6.61) value while the highest concentration (150 mg/mL) recorded the lowest pH (6.24) value. Although significantly (p< 0.05) lower than the 18.75 mg/mL concentration, HPA solutions of 75 and 37.5 mg/mL were not significantly (p>0.05) different from each other in their pH values.
Effect of heat treatment and serial dilution on physical properties of different concentrations of HPA solutions
Heat treatment had a significant (p<0.05) effect on all physical properties (osmolality, pH, and pCO2), with the exception (p> 0.05) of viscosity. Heating HPA solution for 60 minutes increased osmolality, compared to both 30 minutes heating and non-heated HPA solutions. However, the non-heated HPA solutions had higher (p<0.05) osmolality than samples heated for 30 minutes. The pH values of HPA solutions decreased with increased period of heat treatment, while pCO2 increased with prolonged exposure of HPA solutions to heat treatment. The viscosity of HPA solutions remained unchanged with heat treatment, although the value observed for 60 minutes heat treatment was marginally higher than other heat treatments.
The interactions between HPA concentration and heat treatment were significant with regards to osmolality (p<0.01), pH (p<0.01) and pCO2 (p<0.05) of HPA solutions. No such interaction was found for viscosity of the solutions. However, there were marginally higher viscosity values due to the interaction of 150 mg/mL HPA concentration and heat treatments.
Chemical composition of different concentrations of HPA solutions
The results of the effect of heat treatment, concentration of HPA solutions and their interactions on the chemical composition of HPA solutions are presented in Table 4. Across all heat treatments, K+ was not detected in the 150 and 75 mg/mL concentrations of HPA solutions, glucose was not detected at the lowest (9.375 mg/mL) concentration, while lactate was not detected at 18.75 and 9.375 mg/mL concentrations. Meanwhile, Na+ and Cl− were completely absent across all heat treatments and concentrations of HPA solution. Generally, there was an increasing concentration of Ca2+ (p<0.05), K+, glucose, and lactate (p<0.01) with every increase in the concentration of HPA solution, with the exception of acid-base excess, which reduced (p<0.01) as concentration of HPA solution increased. Also, the concentrations of Ca2+, glucose and lactate rose with increase in heating duration, while that of K+ and acid-base excess declined.
Effect of heat treatment and serial dilution on chemical compositions (electrolytes and metabolites) of different concentrations of HPA solutions
The interactions between HPA concentration and heat treatment were significant with regards to the levels of glucose (p<0.05), lactate (p<0.01) and acid-base status (p<0.01). No such interactions were observed for Ca2+ and K+ contents of the HPA solutions.
Protein content and amino acid profiles of different concentrations of HPA
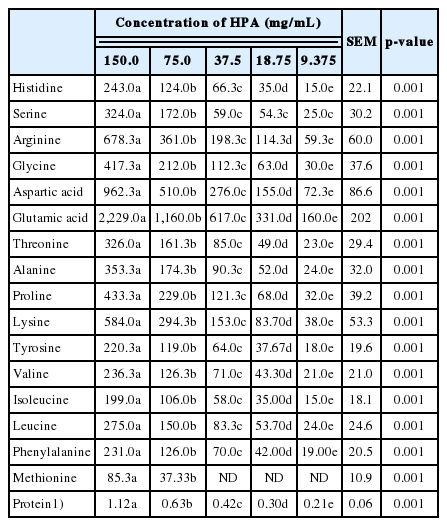
The protein content of HPA solutions decreased (p<0.05) linearly with reduction in the concentrations of HPA solution (Table 5). The highest value was observed at 150 mg/mL concentration, while the lowest value was observed at 9.375 mg/mL of HPA solution. The highest value of protein was recorded at 60 minutes of heat treatment, while there were no differences (p>0.05) between the solutions heated for 30 minutes and the cold solution. Also, protein content increased (p<0.01) with longer duration of heating as well. A significant (p<0.05) interaction was observed between HPA concentration and heat with respect to the protein contents of HPA solutions (data not shown).
The concentrations of amino acids declined (p<0.05) with serial dilution of the solutions but the pattern of concentrations of amino acids was unaffected. Glutamic acid was in the highest concentration, followed by aspartic acid. Of the key indispensable amino acids, lysine was in the highest concentration while methionine was not detected in the lower concentrations of HPA solutions.
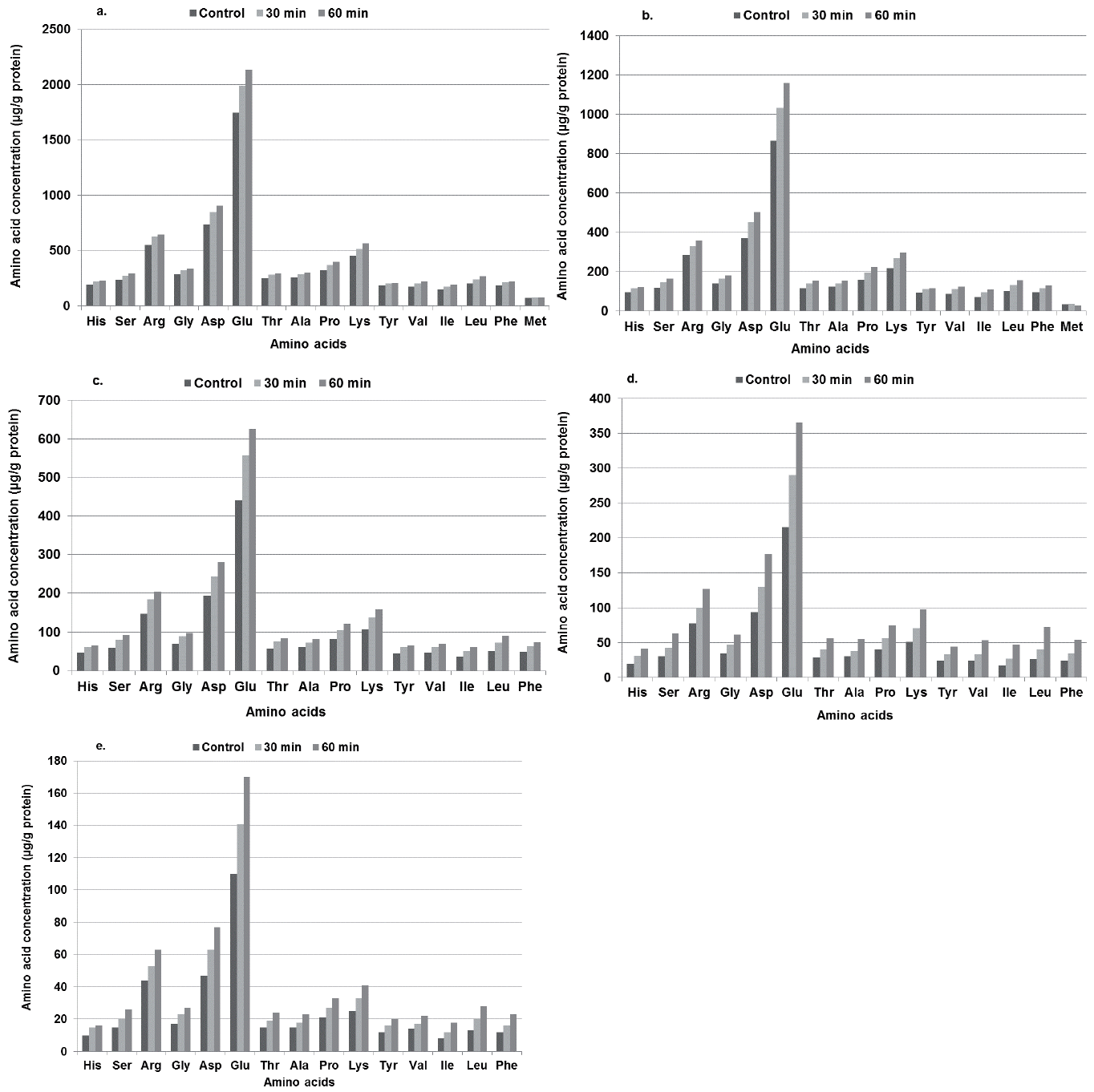
Heat treatment appeared to increase (p<0.05) the free amino acid concentrations of HPA solutions, with concentrations being highest in the solutions heated for 60 minutes and lowest in the non-heated solutions across all amino acids studied. This was observed in all concentrations of HPA solutions (Figure 1a–1e).
DISCUSSION
Physical properties of amniotic fluid from late-incubation eggs and HPA solutions
In this study, the weight of eggs from different strains of broiler breeders was different before incubation, and on day 17 of incubation. The results showed that Cobb 500 has heavier eggs on day 17 of incubation. However, this may be as a result of the effect of initial differences in the weight of eggs on arrival, which was not initially considered as a factor that may influence amniotic fluid characteristics at late incubation. However, it is known that the age of broiler breeders affects egg weight [17,18]. Eggs used in this study were from broiler breeders of different ages.
Irrespective of differences in initial and late-incubation egg weights, the percentage egg weight losses obtained in this study for each strain were similar to what has been previously reported in literature as optimal for best chance of hatching success and good chick quality at hatch [19]. The marginal differences obtained in egg weight loss in this study could therefore be attributed to differences in age of breeders, and also but not necessarily, to the differences in strain.
There were no significant differences observed in physical properties of late-incubation amniotic fluids from different broiler strains with the exception of viscosity. The osmolality values obtained from this study are high and identical to those obtained by Bolin [20], although from amniotic fluid of eggs exposed to low humidity on day 18 of incubation. This suggests that the strain and age of broiler breeders, and differences in egg weight do not affect the physical properties of late-incubation egg amniotic fluid, and so may not be a factor of concern in carrying out IOF. The reason for the high osmolality values in the current study is unclear but may be attributed to a not assessed but possible low humidity condition within the incubator. If the osmolality of the amniotic fluid surpasses that of the body fluids of the developing embryo, the embryo will dehydrate and may not be able to complete the process of hatching.
Our result shows that amniotic fluid from late-incubation eggs from Cobb 500 is more viscous than amniotic fluid from Ross 308, implying that the Cobb 500 embryo may be able to consume the amniotic fluid at late-incubation more easily and faster. It is known that sero-amniotic connection formed around day 12 of incubation allows the movement of albumen protein and water into the amniotic fluid [1]. Carinci and Manzoli-Guidott [21] related increased protein content of amniotic fluid to increased viscosity. This may be the explanation for the difference observed, suggesting that more albumen-containing protein and water may have moved into the amniotic fluid in Cobb 500 eggs. If the fluid is supplemented with nutrients in ovo, they may then have more utilizable nutrients than the Ross 308 embryos.
To understand and align the properties of IOF supplement to that of late-incubation egg amniotic fluid, this study investigated the physical properties of a potential IOF product, produced by preparing a solution from a mixture of a processed soy protein, HPA, obtained from Hamlet Protein in deionised water at different levels of concentration. The objective was to attempt to identify or develop a novel product that optimises embryonic development or reduce the known adverse effects of currently available IOF supplements, especially with regards to low hatchability. This product should be close to the amniotic fluid in terms of physico-chemical properties. Currently, there are no available reports in literature with detailed study on the physical properties of the various IOF supplements available, either commercially or at trial levels.
Generally, osmolality was significantly higher in the highest concentration of HPA solution within each heat treatment groups and across serial dilutions. This suggests that, while heat treatment may have resulted in solubilizing more of the HPA in the solution, thereby increasing the osmolality, the more the product per mL of water, the higher the osmolality of the final solution. It seems that osmolality of IOF supplements would vary widely, depending on formulation and nutrient contents. A study by Ferket et al [22] recommended IOF supplement with 400 to 600 mOsm) as the optimal range for IOF solutions, based on their observations in turkeys. Uni and Ferket [3] reported a decreasing hatchability with increasing osmolality (above 800 mOsm) when a sodium chloride-based IOF supplement was administered in ovo in chicken eggs during late-term embryonic development.
The viscosity of the test product in this study followed similar trend as osmolality with respect to the effects of heat treatment, concentrations of HPA solution and their interactive effects. The only difference is that viscosity consistently increased with increased heat treatment at each level of concentration of HPA solution. However, the values obtained were much lower than the viscosity values for amniotic fluid of late-incubation egg.
However, pH rather increased and tended toward neutrality with lesser concentration of HPA solution, and maintained this trend irrespective of degree of heat treatment across each level of concentration. The higher concentration of HPA solutions were more acidic. The pH values were all marginally lower than the pH of amniotic fluid of eggs. Whether this would be a limitation for use of this product as an IOF supplement is not known until it is further investigated. Most studies on IOF do not report the pH values of the IOF supplements used [23,24]. The first attempt to report the pH of an IOF supplement was by de Oliveira [14], who used an IOF solution of pH 6.99 in turkey eggs. The pH status of an IOF supplement may be an overlooked property that may be imposing a constraint to the response of IOF as a technique for the poultry industry. With all these having been stated, it is important to consider whether breed differences and age of breeder will affect what these limits should be for IOF supplements.
There is no report of the pCO2 status of IOF supplement elsewhere in literature as at the time of this study. Our study shows that the pCO2 of HPA solution varied with heat treatment and concentration of HPA solution. In this study, we observed that with increasing heat treatment, pCO2 increased up to 30 minutes of heat treatment but no difference was observed beyond 30 minutes of heat treatment. Serial dilution affected pCO2 status of HPA solutions, tending to increase with each dilution. In other species, elevated environmental pCO2 have been implicated for negative impacts. Sigwart et al [25] showed that elevated pCO2 drives lower growth and increased calcification in early life in the cuttlefish, Sepia officinalis. They reported that embryonic growth was reduced and hatching delayed under elevated pCO2, both at environmentally relevant levels (1.05 mmHg pCO2) and extreme conditions (3.00 mmHg pCO2). Although the pCO2 tolerance levels/limits of a developing chicken embryo may be higher than reported by their study, it gives an indication of the potential effect of a high pCO2 IOF supplement on a developing embryo if administered in ovo.
The physical properties of HPA solution are affected by concentration of HPA in the solution, heat treatment and the interaction between both factors. The physical properties of HPA solutions are lower but comparable to those of late-incubation egg amniotic fluid, especially in terms of osmolality and pH, suggesting that HPA solution may not pose any danger to developing embryos when used as an IOF supplement. However, a longer duration of heating beyond 60 minutes may be required to bring the viscosity and pCO2 of HPA solutions closer to those of late-incubation egg amniotic fluid. It seems that to develop an IOF product from a protein source that would possess osmolality characteristics close to that of the amniotic fluid, some factors such as the concentration of the product in a solution, heat treatment and the interactions between both factors need to be put into consideration in developing a method of producing or processing such product.
Chemical composition of amniotic fluid from late-incubation egg and HPA solutions
The results showed that with the exception of K+ there are no significant differences in the composition of the amniotic fluid from late-incubation eggs, irrespective of differences in strains, age of broiler breeders and egg weights on setting and on day 17 of incubation. Interestingly, Na+ is the most abundant electrolyte in amniotic fluid of late-incubation egg, while Ca2+ is the least abundant. This is contrary to Romanoff and Romanoff [2], who reported Cl− as the most abundant electrolyte, although their data were from eggs at 15 days of incubation.
Our findings show that glucose and lactate are present in small quantities, compared to the mineral electrolytes in the amniotic fluid of late-incubation eggs. This agrees with the finding that carbohydrates are present at very low levels in the egg (<1%) at oviposition and the total amount decreases even further throughout incubation [26]. However, this may be an indication that glucose and lactate are used up in anaerobic glycolysis in the muscles during late incubation. Furthermore, environmental conditions may be a factor in lowered glucose level as Molenaar et al [27] had previously shown that hepatic glycogen stores at day 18 of incubation, just before the hatching process started, were reduced by 30% when eggshell temperature was elevated from 37.8°C to 38.9°C from day 7 of incubation onward. It would appear that glycogen may not play a significant role in the development of the embryo in view of its low level in the amniotic fluid. Although not significantly different, glucose concentration in amniotic fluid from Ross 308 eggs was almost double what was obtained in Cobb 500 eggs. While a previous report [2] indicated identical amount of glucose in chicken amniotic fluid on day 17 of incubation, nothing was mentioned of lactate in the amniotic fluid. In allantoic fluid, lactic acid was only found after day 9 of incubation. Results from the present study provide insight for IOF administration to avoid toxicity or deficiency that may result from use of IOF nutrients or supplements.
The HPA solutions seem to be relatively low in mineral electrolyte contents. Unlike in amniotic fluid of late-incubation egg, HPA solutions are deficient in Na+ and Cl− as these were not detected, irrespective of heat treatments or concentrations of HPA solutions. This may be as a result of low levels of these two minerals in soybean, the main source of HPA. Although there was some effect of heat treatment and change in concentrations of HPA solution, these effects were negligible. Potassium appeared to be very high in the higher concentrations of HPA solution, and was beyond the detection range of the equipment. This is expected as the study by Batal et al [28] reported higher levels of potassium than calcium in soybean meal. Physiological saline (sodium chloride) has traditionally been assumed to be a good carrier for injected nutrients and has been used in the past [3,5,14,22]. The mid-level concentration of HPA solution (37.5 mg/mL) is similar to the amniotic fluid of late incubation egg in potassium content, especially the Ross 308 eggs. This means that the solution may be able to meet the potassium requirement of the developing embryo or support the role played by amniotic potassium in embryonic development.
The carbohydrate metabolite contents of HPA solution were significantly affected by heat treatment and concentration of HPA. Glucose was not detected in the lowest HPA concentration, while lactate was not detected in the two lowest concentrations. While the effect of the treatments on glucose and lactate did not follow any specific pattern, lactate was most abundant at the highest concentration but was not affected by heat treatment. Longer heat duration and increasing concentration, especially at 37.5 mg/mL, brings the glucose content of HPA close to that of amniotic fluid from Ross 308 eggs, and higher than in Cobb 500 eggs. Similarly, heating HPA solutions for 30 minutes results in higher lactate than is found in late incubation amniotic fluid. Supplementing HPA as an IOF product may mean providing extra glucose, which may serve as energy substrate and is crucial for hatchling development [29] and lactate to the developing embryo, adding to what is already available to it in the amniotic fluid.
However, the concentration and volume of the solutions being injected must be taken into consideration to best determine the supportive roles of the various salts and metabolites on embryonic growth and development as well as post-natal growth performance and their associated effects on the biochemical profile of the blood [10].
Protein content and amino acid profiles of amniotic fluid from late-incubation eggs and HPA solutions
Very little is known about the protein composition of amniotic fluid of late-incubation eggs. In this study, protein content of amniotic fluid from late-incubation eggs from different strains of broiler breeders did not differ, although, values were marginally higher in Ross 308 and this result reflected in the amino acid profile. Romanoff and Romanoff [2] reported a higher protein content than was reported in this study in amniotic fluid during late-incubation. The reasons for the variations are not known but may be as a result of breeder nutrition, although breeds have changed over the years, differences in method of determination or condition in which eggs were incubated. The lower levels of protein observed in amniotic fluid at late-incubation may be an indication of the utilization of protein by the developing embryos. Shafey et al [30] reported that the embryo relies on the metabolism of carbohydrates and protein in the first two weeks of life, and utilizes amino acids for tissue growth at a higher rate during the second half of incubation.
There is very little in literature on the amino acid profiles of amniotic fluids from late-incubation eggs. Our results show that there is no significant difference in the amino acid profile of the amniotic fluids from late-incubation eggs from different broiler breeders of different ages. However, the amniotic fluid from late-incubation eggs obtained from Ross 308 had marginally higher values than Cobb 500 across all the sixteen amino acids analysed. Amniotic fluid from late-incubation egg is richest in non-essential glutamic and aspartic acids. Glutamic acid is involved actively in the removal of oxidants and regulation of immune response [31], while aspartic acid is considered a major energy substrate for the synthesis of purine and pyrimidine, hence crucial for the proliferation of lymphocytes [32]. Of all essential amino acids, the 5 most abundant in descending order are leucine, lysine, valine, phenyalanine, and isoleucine.
The overall goal of IOF is to provide adequate nutrients to the growing embryo. Information on the amino acid profiles of the amniotic fluid of late-incubation egg will be relevant in developing a suitable amino acid in ovo supplement with equivalent amino acid profile to that of the amniotic fluid. Al-Murrani [33] reported that amino acid administration in ovo in broiler breeder eggs, equivalent to the profile of embryo amino acids, increased chick weight from hatch to day 56, while Ohta et al [34] suggested that protein utilization, rather than individual amino acid utilization, of embryo may be improved when an amino acid solution, identical to the amino acid makeup of the egg, is injected at day 7 of incubation.
The protein contents of HPA solution were affected by heat treatment and concentration of HPA, as well as the interactions between both factors. Protein was mostly abundant in the highest concentration of HPA and decreased with each consecutive serial dilution. Generally, the quantity of protein in the solutions was less than what was observed in the amniotic fluid of the eggs. This was not expected since HPA is mostly soybean. However, it is important to mention that the solution used was only a supernatant of the mixture of HPA and MQ water. It is possible that only a fraction of amino acids in HPA were extracted into water during the process of making the solutions.
The amino acid profiles of HPA indicate relatively high levels of lysine, one of the most important amino acids in poultry nutrition but methionine was not detected in the lower concentrations of the supplement. The product also contains a high level of glycine, which has been linked to development of immune function in poultry [35] and also involved in collagen production [36], and proline, a key component of hydroxyproline, the main protein in gelatin [37]. Since collagen is essential for tissue development, this product may potentially be useful as an IOF supplement. The amino acid profile of the highest concentration of HPA solution is close to the composition of amino acid solution injected into hatching eggs by Ohta et al [38], although both varied in quantity of each amino acid, with the exception of cysteine and tryptophan, which were not measured in this study. An adequate amount of amino acids must be available to meet the increased physiological demands of the embryo, especially in the last stage of incubation [30]. It is however, not certain what should be the optimum level of the supplement that can be used in IOF in order to meet the amino acid requirements of a developing chicken embryo during late-incubation stage. Increasing the duration of exposure of supplement to heat treatment increased the concentration of amino acids but it is not certain if the availability of amino acid will be affected by this heat treatment. The data obtained cannot be directly linked to the requirements of embryos in the final phase of incubation.
CONCLUSION
The amniotic fluid of late-incubation eggs from different broiler breeders of different ages do not differ much in physical properties and chemical compositions apart from weights, viscosity and K+. Their amino acid profiles were also similar. This, therefore, excludes these factors as possible constraints to be considered when planning IOF. The amino acid profile of the amniotic fluid indicates that it is rich in glutamic acid, aspartic acid, leucine and lysine, which may be major requirement in the late developing embryo. The HPA solution is relatively lower in comparison to the amniotic fluid of late-incubation egg in all physical properties studied. The test product contains a range of amino acids that reflect the profile of the parent product, soybean meal, but at higher concentrations. The amino acid profile of HPA solution is comparable to the amino acid supplements used for IOF in terms of composition. It seems that the most reliable HPA solution for IOF is the highest concentration, heated for 60 minutes. Actual tests will need to be conducted to determine the optimum level of inclusion of the product as an IOF supplement and response of chicks in later life.
ACKNOWLEDGMENTS
We would like to thank the University of New England, Armidale and Hamlet Protein, Denmark for their financial support.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.