In vitro Solubility of Copper(II) Sulfate and Dicopper Chloride Trihydroxide for Pigs
Article information
Abstract
This study was conducted to determine the solubility of copper (Cu) in two sources of copper(II) sulfate (CuSO4) including monohydrate and pentahydrate and three sources of dicopper chloride trihydroxide (dCCTH) including α-form (dCCTH-α), β-form (dCCTH-β), and a mixture of α- and β-form (dCCTH-αβ) at different pH and a 3-step in vitro digestion assay for pigs. In Exp. 1, Cu sources were incubated in water-based buffers at pH 2.0, 3.0, 4.8, and 6.8 for 4 h using a shaking incubator at 39°C. The CuSO4 sources were completely dissolved within 15 min except at pH 6.8. The solubility of Cu in dCCTH-α was greater (p<0.05) than dCCTH-β but was not different from dCCTH-αβ during 3-h incubation at pH 2.0 and during 2-h incubation at pH 3.0. At pH 4.8, there were no significant differences in solubility of Cu in dCCTH sources. Copper in dCCTH sources were non-soluble at pH 6.8. In Exp. 2, the solubility of Cu was determined during the 3-step in vitro digestion assay for pigs. All sources of Cu were completely dissolved in step 1 which simulated digestion in the stomach. In Exp. 3, the solubility of Cu in experimental diets including a control diet and diets containing 250 mg/kg of additional Cu from five Cu sources was determined during the in vitro digestion assay. The solubility of Cu in diets containing additional Cu sources were greater (p<0.05) than the control diet in step 1. In conclusion, the solubility of Cu was influenced by pH of digesta but was not different among sources based on the in vitro digestion assay.
INTRODUCTION
Copper (Cu) is essentially required for pigs to serve many functions in the body such as enzyme activations, immune functions, and hemoglobin synthesis (Hill and Spears, 2001). The NRC (2012) suggested that the requirements of Cu were 5 to 6 mg/kg for weanling pigs and 3 to 4 mg/kg for growing-finishing pigs in order to maintain normal metabolism. Many studies have reported that pharmacological concentrations of Cu in diets, ranged from 100 to 250 mg/kg, improved growth performance of weanling pigs (Cromwell et al., 1998; Hill et al., 2000; Veum et al., 2004; Pérez et al., 2011; Shelton et al., 2011) and growing pigs (Cromwell et al., 1978; Zhao et al., 2014). Therefore, dietary Cu sources are generally used to enhance the growth performance of pigs especially in the post-weaning phase. However, the action mechanism of the growth-promoting effect of Cu is still debatable.
Many studies have been conducted to determine the possible mechanisms for growth-promoting effects of dietary Cu. Højberg et al. (2005) and Namkung et al. (2006) suggested that pharmacological concentration of dietary Cu reduced population of pathogenic bacteria in the gut and subsequently increased the growth performance of weanling pigs. In addition, Shurson et al. (1990) and Radecki et al. (1992) reported that dietary Cu improved the gut health of weanling pigs. On the other hand, Zhou et al. (1994) reported that intravenous injection of Cu improved the growth performance of weanling pigs. Whether dietary Cu improves the growth performance by affecting the condition of gastrointestinal tract, by promoting the systemic action, or both, liberation of Cu from the compound is critical to elucidate its growth-promoting mechanisms in pigs, which may be partially explained by in vitro solubility.
Copper(II) sulfate (CuSO4) is widely used in swine diets as growth promoter and dicopper chloride trihydroxide (dCCTH), commonly known as tribasic copper chloride, is also used in swine diets to improve the growth performance of pigs (Shelton et al., 2011). Comparison among the in vitro solubility of Cu in these two products was conducted in the previous experiments (Pang and Applegate, 2006; 2007). However, to the best of our knowledge, the solubility of Cu in these two products has not been evaluated under the condition of pigs. Moreover, the solubility of Cu in CuSO4 may be affected by the degree of hydration and that of Cu in dCCTH may also be affected by the structure of its molecule. Therefore, the objective of this study was to determine the solubility of Cu in two sources of CuSO4 including monohydrate (CuSO4·H2O) and pentahydrate (CuSO4·5H2O) and three sources of dCCTH including α-form (dCCTH-α), β-form (dCCTH-β), and a mixture of α- and β-form (dCCTH-αβ) at different pH and a 3-step in vitro digestion assay for pigs.
MATERIALS AND METHODS
Exp. 1
Exp. 1 was conducted to determine the solubility of Cu in five sources of Cu at pH 2.0, 3.0, 4.8, and 6.8 buffers during 4-h incubation. Two sources of CuSO4 including CuSO4·H2O and CuSO4·5H2O and three sources of dCCTH including dCCTH-α, dCCTH-β, and dCCTH-αβ were analyzed in triplicate. The molecular structure of dCCTH was differentiated by its structure of surface, which can be observed by scanning electron microscopy (Figure 1). The dCCTH-αβ consisted of 30% of α-form and 70% of β-form. Each Cu source was weighed to contain 83.4 mg Cu/L in the buffer solution and put into a 500-mL conical flask. The concentration of Cu was the estimated concentration of Cu in digesta of pigs fed the diet containing 250 mg/kg of Cu and consumed water with a ratio relative to consumed feed at 2:1 (Pang and Applegate, 2007). A buffer solution (250 mL) was added to a conical flask. Buffers were prepared by adding 1 M HCl for pH 2.0 and 3.0, 10% acetic acid for pH 4.8, and 0.1 M NaOH for pH 6.8 to distilled water. Buffers containing Cu sources were immediately incubated in a shaking incubator at 39°C for 4 h. During the incubation, a 2 mL aliquot was collected at 15 and 30 min and 1, 2, 3, and 4 h after the beginning of the incubation. Each aliquot of sample was analyzed for the Cu concentration using an atomic absorption spectrophotometer (AAS; novAA300, Analytik Jena AG, Jena, Germany). Each subsample was diluted 1:5 with distilled water when the expected concentration of Cu in subsample exceeded the detection limit of AAS. The solubility of Cu was then determined by the following equation:
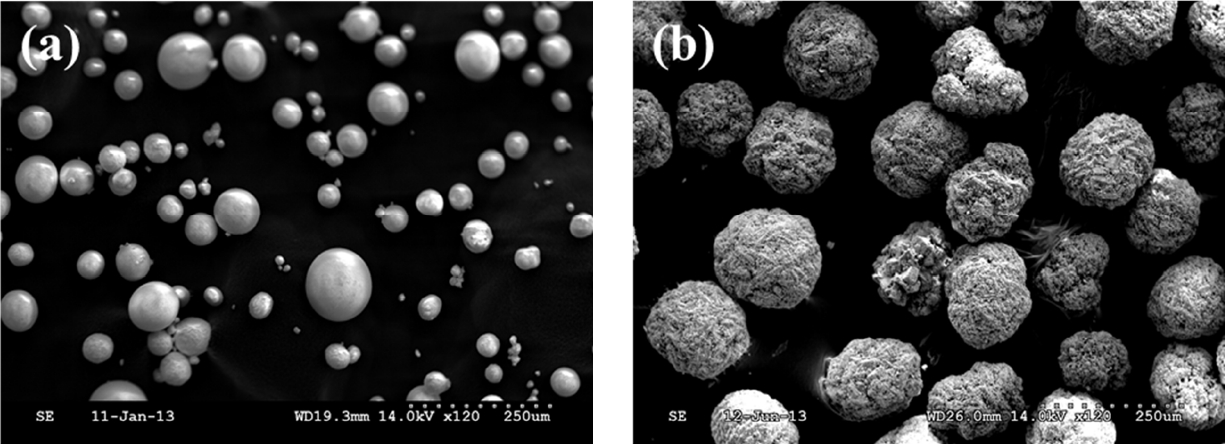
Scanning electron microscopy images of dicopper chloride trihydroxide consisted of α-form (a) and β-form (b).
Exp. 2
Exp. 2 was conducted to determine the solubility of Cu in five sources of Cu during the 3-step in vitro digestion assay for pigs. Five sources of Cu including two sources of CuSO4 and three sources of dCCTH were the same as in Exp. 1 and analyzed in triplicate.
In vitro digestion assay consisted of 3 steps to simulate the digestion in the stomach, small intestine, and large intestine, respectively, based on the procedure of Boisen and Fernández (1997) with several modifications. Prior to the in vitro analysis, the final volume of solution (approximately 64.4 mL) was adjusted to 250 mL, and the volume of solutions used in each step was calculated. Each Cu source was weighed to contain 83.4 mg Cu/L in the 250 mL of the final solution and put into a 500-mL conical flask. In step 1, sodium phosphate buffer solution (0.1 M, pH 6.0) and 0.2 M HCl solution were added in flasks containing samples of interest. The estimated pH of mixed solution in the flask was 2.0. Thereafter, freshly prepared pepsin solution (25 mg/mL; ≥250 units/mg solid, P7000, Pepsin from porcine gastric mucosa, Sigma-Aldrich, St. Louis, MO, USA) and chloramphenicol (C0378, Chloramphenicol, Sigma-Aldrich, USA) solution (5 g/L ethanol) was added. Each test flask was sealed and incubated in a shaking incubator at 39°C for 2 h. In step 2, sodium phosphate buffer solution (0.2 M, pH 6.8), 0.6 M NaOH solution, and freshly prepared pancreatin solution (100 mg/mL; 4×USP, P1750, Pancreatin from porcine pancreas, Sigma-Aldrich, USA) were added in the flasks, immediately after the step 1 incubation. The estimated pH of mixed solution in the flask was 6.8. The test flasks were then incubated in a shaking incubator at 39°C for 4 h. In step 3, ethylenediaminetetraacetic acid solution (0.2 M) was added in the flasks, and pH was adjusted to 4.8 by adding 30% acetic acid. Viscozyme (cellulolytic enzyme mixture, V2010, Viscozyme L, Sigma-Aldrich, USA) was added to simulate the microbial fermentation in the large intestine. The test flasks were then incubated in a shaking incubator at 39°C for 18 h. An aliquot of sample (2 mL) was collected at the end of each step and subsequently diluted 1:10 or 1:20 with distilled water. The concentration of Cu in each subsample was analyzed using AAS. The solubility of Cu was calculated by the same equation in Exp. 1.
Exp. 3
Exp. 3 was conducted to determine the solubility of Cu in six experimental diets during the 3-step in vitro digestion assay for pigs. A control diet mainly containing corn and soybean meal was formulated (Table 1). Dried whey and fish meal were added in order to prepare the control diet for weanling pigs. The calculated concentration of Cu in the control diet was 17.9 mg/kg. Five additional diets were formulated to contain 250 mg/kg of additional Cu as CuSO4·H2O, CuSO4·5H2O, dCCTH-α, dCCTH-β, and dCCTH-αβ, respectively, at the expense of corn. Diets were formulated to meet or exceed the nutrient requirement estimates for 10 to 20 kg weanling pigs (NRC, 1998). The in vitro digestion assay was conducted based on the procedure of Boisen and Fernández (1997) without adjustment of the final volume. Thus, 500 mg of experimental diets were analyzed in triplicate. A 2-mL aliquot collected at the end of each step was analyzed for the Cu concentration using AAS. The solubility of Cu was calculated by the same equation in Exp. 1.
Statistical analysis
Data were analyzed by analysis of variance using general linear model procedure of SAS (SAS Inst. Inc., Cary, NC, USA). In Exp. 1 and 2, five sources of Cu were used as the independent variable. Experimental diets were used as the independent variable in Exp. 3. Least squares means for the solubility of Cu were calculated and separated by PDIFF option with the Tukey’s adjustment. The experimental unit was the replicate and statistical significance was set at p<0.05.
RESULTS
Exp. 1
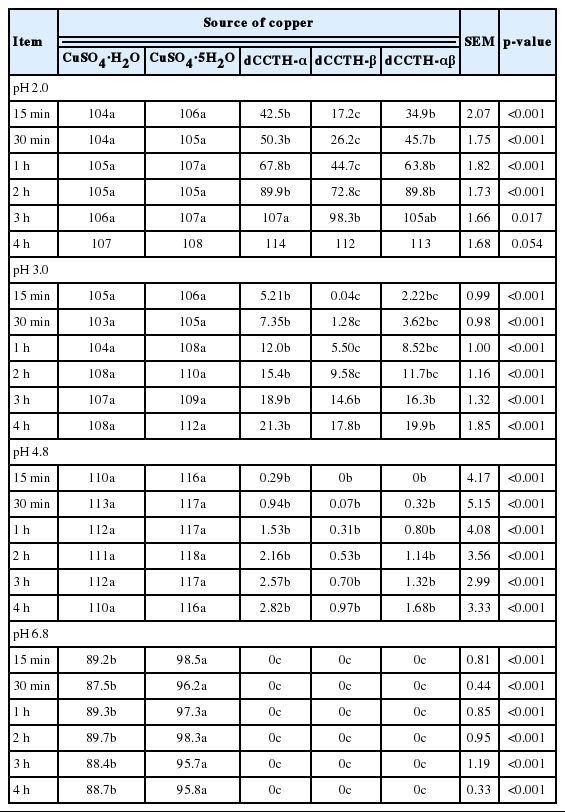
The CuSO4 sources were completely dissolved within 15 min except at pH 6.8 (Table 2). At pH 2.0, values for the solubility of Cu in dCCTH-α and dCCTH-αβ were greater (p<0.05) than dCCTH-β during the 2-h incubation. On 3 h of incubation, the solubility of Cu in dCCTH-β was less (p<0.05) than that of Cu in dCCTH-α, but was not different from that of Cu in dCCTH-αβ. At the end of the incubation, the solubility of Cu was not different. In pH 3.0 buffer, the solubility of Cu in dCCTH-β was less (p<0.05) than that of Cu in dCCTH-α, but was not different from dCCTH-αβ until 2 h of incubation. After 3 h, the solubility of Cu was not different among dCCTH sources. However, at the end of the incubation, values for the solubility of Cu in dCCTH sources were less than 22%, and were also less (p<0.05) than those of Cu in CuSO4 sources. At pH 4.8, there were no significant differences among the solubility of Cu in dCCTH sources during the 4-h incubation. At the end of the incubation, values for the solubility of Cu in dCCTH sources were less than 3%, and were also less (p<0.05) than those of Cu in CuSO4 sources. In pH 6.8 buffer, the solubility of Cu in CuSO4·5H2O was greater (p<0.05) than that of Cu in CuSO4·H2O during the overall incubation. The concentrations of Cu in samples collected from dCCTH sources were not detected during the overall incubation.
Exp. 2
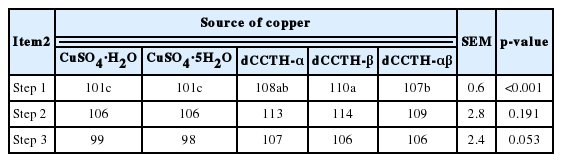
Even though Cu in both CuSO4 and dCCTH sources were completely dissolved in step 1, significant difference was observed (p<0.001) among the solubility of Cu (Table 3). However, values for the solubility of Cu in five Cu sources were not different in step 2 and 3.
Exp. 3
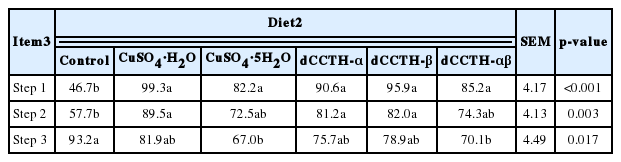
Values for the solubility of Cu in diets containing additional Cu sources were greater (p<0.05) than that of Cu in the control diet in step 1 (Table 4). However, after the step 2 incubation, values for the solubility of Cu in diets containing CuSO4·H2O, dCCTH-α, and dCCTH-β were greater (p<0.05) than that of Cu in the control diet, but were not different from those of Cu in diets containing CuSO4·5H2O and dCCTH-αβ. At the end of the in vitro digestion assay, the solubility of Cu in the control diet was greater (p<0.05) than the diet containing CuSO4·5H2O and dCCTH-αβ, but was not different from the diet containing CuSO4·H2O, dCCTH-α, and dCCTH-β.
DISCUSSION
Although Cu has been used to improve the growth performance of both pigs (Cromwell, 2001) and poultry (Leeson, 2009), accurate mechanisms of Cu are not clearly identified. Previous studies suggested that growth-promoting effects of dietary Cu might be attributed to both antibacterial properties (Højberg, 2005; Namkung et al., 2006) and systemic actions (Zhou et al., 1994). To act as an antimicrobial agent, Cu should be dissolved in the gastrointestinal tract. Zevenhuizen et al. (1979) reported that the growth of Cu-sensitive bacteria including Escherichia coli B, Klebsiella aerogenes H, and Alcaligenes Ad-4 was inhibited in Cu-containing media in which Cu ion was derived from CuSO4 and ranged from 0.06 to 0.6 mg/kg. Pang et al. (2009) also reported from an in vitro experiment that the number of Escherichia coli in the ileal digesta of broiler decreased quadratically when incubated with the diets containing graded concentration of Cu up to 250 mg/kg as CuSO4·5H2O. On the other hand, to act systemically so as to improve the growth performance of pigs (Zhou et al., 1994), Cu should be dissolved for absorption in the gastrointestinal tract (Hill and Spears, 2001). Therefore, dietary Cu sources may be dissolved in appropriate site of the gastrointestinal tract in order to improve the growth performance, and difference in solubility of Cu among Cu sources at various pH conditions can partially represent their properties as the growth-promoter.
In the current experiment, values for the solubility of Cu greater than 100% were commonly observed. These observations may be due to the analytical errors in the concentration of Cu. Because the detection limit of AAS ranged from 0 to 20 mg/L, subsamples collected in each time period were diluted in order to appropriately analyze the concentration of Cu, which may cause the errors when determining the solubility of Cu. Moreover, in Exp. 1, a 2 mL of aliquot was consistently collected from the 250 mL buffer solution containing each Cu source, and therefore, the volume of buffer was gradually reduced whenever the subsample was collected. On the contrary, in Exp. 2 and 3, the solutions involved in each digestion step were added after the prior step was finished and a 2 mL of aliquot was collected. Although the total volume of mixed solutions was adjusted when calculating the concentration of Cu at the end of each step, analytical errors may exist due to the increased number of solutions used in digestion. Due to the cumulative addition of solutions in each step, values for the solubility of Cu in diets containing Cu sources in Exp. 3 may be reduced in step 2 and 3.
In Exp. 1, the solubility of Cu in CuSO4·5H2O was in agreement with the previously reported values (Pang and Applegate, 2006; 2007) except when incubated in pH 6.8 buffer. In spite of greater pH condition, values for the solubility of Cu in CuSO4·5H2O incubated in pH 6.8 buffer were greater than the values reported in Pang and Applegate (2006; 2007), which were 87.9% and 75.5%, respectively. In addition, values for the solubility of Cu in dCCTH sources observed in Exp. 1 were less than the values reported in Pang and Applegate (2006; 2007). This may be due to the fact that buffers used in the present experiment were different from those used in the previous reports (Pang and Applegate, 2006; 2007), which used 0.2 mM Glycine-HCl buffer for pH 2.5 and 0.2 mM sodium acetate buffers for pH 5.5 and 6.5.
Copper(II) sulfate pentahydrate is dominantly used in swine diets (Shelton et al., 2011) and it has been used as a standard Cu source to estimate relative bioavailability of Cu in Cu sources for poultry (Guo et al., 2001; Miles et al., 2003). However, there is an increasing attention on the use of CuSO4·H2O as an alternative to CuSO4·5H2O because of its several benefits including less caking problems and better mixability in the mineral premix (Kim and Kil, 2015; Kim et al., 2016). In the present experiment, values for the solubility of Cu in CuSO4·H2O were not different from CuSO4·5H2O in pH 2.0, 3.0, and 4.8 buffers. Although values for the solubility of Cu in CuSO4·5H2O were greater than those in CuSO4·H2O during the 4-h incubation at pH 6.8, it is likely that bioavailability and growth-promoting effects of CuSO4·H2O is similar to CuSO4·5H2O because both Cu sources have a similar chemical structure and are highly soluble in various pH conditions.
In pH 3.0 buffer, values for the solubility of Cu in dCCTH sources were less than 22% during the 4-h incubation; in addition, solubility was less than 3% when incubated in pH 4.8 buffers during the 4-h incubation. Copper in dCCTH sources were not dissolved in pH 6.8 buffers for 4 h. These observations were in agreement with Pang and Applegate (2006; 2007) who reported that the solubility of Cu in dCCTH was decreased as the pH of buffer increased. Among dCCTH sources, dCCTH-β was less soluble than dCCTH-α during 3-h incubation at pH 2.0 and during 2-h incubation at pH 3.0. Although dCCTH-αβ contained more β-form than α-form, values for the solubility of Cu in dCCTH-αβ were not different from dCCTH-α. To the best of our knowledge, there is limited information about the effects of molecular structure of dCCTH on the solubility of Cu. Therefore, the reason why the solubility of Cu in dCCTH-β was less than dCCTH-α in low pH conditions remains unclear; however, it may be speculated that the solubility of Cu in dCCTH sources were affected by the pH of digesta greater than CuSO4 sources. Further research is needed to determine whether the structure of dCCTH affects the bioavailability and growth-promoting effects of Cu.
In Exp. 2, all Cu sources were completely dissolved in step 1 of in vitro digestion assay. Compared to the result of Exp. 1, dCCTH sources were more soluble in sodium phosphate-based buffer than in water-based buffer; in addition, pepsin may increase the solubility of Cu in dCCTH sources. In Exp. 3, most of Cu in diets containing Cu sources were dissolved in step 1 of in vitro digestion assay. These observations suggest that Cu sources used in this experiment were readily available in the gastrointestinal tract of pigs. Pang and Applegate (2007) reported that the in vitro solubility of Cu might not accurately represent the bioavailability of Cu in broilers. Although it is impossible to exactly mimic the in vivo digestion in the in vitro digestion assay due to the physiological and environmental factors (Boisen and Eggum, 1991), the in vitro digestion procedure has been employed in the evaluation of feed ingredients (Park et al., 2012; Cervantes-Pahm et al., 2013), exogenous enzyme tests (Kong et al., 2015; Park et al., 2016), and mycotoxin sequestering agents (Kong et al., 2014). In the present work, as all Cu sources were dissolved in the solutions prepared to simulate the digestive tract of pigs, the solubility of Cu determined in the in vitro digestion assay for pigs may represent the bioavailability of Cu in Cu sources for pigs.
In Exp. 3, Cu in the control diet was less soluble in step 1 than that in diets containing additional 250 mg/kg of Cu. This may be due to the smaller amount of Cu in the control diet compared with other diets. Dissolution of Cu in the control diet may be inhibited by other feed ingredients because the proportion of Cu is less than other diets. Significant differences were not observed in the solubility of Cu among diets containing Cu sources in all steps, which may indicate that bioavailability of Cu might not be different among Cu sources. Cromwell et al. (1998) reported that both CuSO4 and dCCTH addition improved the growth performance of weanling pigs and the growth performance of pigs fed diets containing pharmacological concentrations of CuSO4 were not different from that of pigs fed diets containing the same concentrations of dCCTH. However, Shelton et al. (2011) observed better growth performance of weanling pigs fed the diet containing 125 mg/kg of Cu as CuSO4 compared with that of weanling pigs fed the diet containing the same concentration of Cu as dCCTH. Pang et al. (2009) also reported that the population of lactobacilli increased and that of Escherichia coli decreased with increased concentration of CuSO4·5H2O, but the effects of dCCTH on the microbial populations were not observed. Further research is needed to determine the relative bioavailability of dCCTH compared with CuSO4.
In conclusion, CuSO4·H2O and CuSO4·5H2O were completely dissolved at pH 2.0, 3.0, and 4.8 within 15 min. Values for the solubility of Cu in CuSO4 sources were greater than those of Cu in dCCTH sources during 4-h incubation at pH 3.0, 4.8, and 6.8. The solubility of Cu in dCCTH-α was greater than that in dCCTH-β but was not different from that in dCCTH-αβ during 3-h incubation at pH 2.0 and during 2-h incubation at pH 3.0. Copper sulfate sources and dCCTH sources were completely dissolved during the step 1 of in vitro digestion assay. Significant differences were not observed among the solubility of Cu in diets containing Cu sources during the in vitro digestion assay, but the solubility of Cu in the control diet was less than that of Cu in diets containing Cu sources in step 1. These results indicated that CuSO4 sources are more soluble than dCCTH sources and the solubility of dCCTH sources are affected by both pH of digesta and their molecular structure.
ACKNOWLEDGMENTS
This paper was supported by the Rural Development Administration (Republic of Korea; PJ010932).