Effects of four dim vs high intensity red color light regimens on growth performance and welfare of broilers
Article information
Abstract
Objective
Broilers show clear preference towards red color light (RL). However setting of an optimum light intensity is difficult since dim intensities that favor growth reduce welfare. This experiment was conducted to test the most effective RL intensity regimen (Dim [5 lux; DI] vs high [320 lux; HI]) in combination applied at different growth stages that favors for both performance and welfare.
Methods
Complete randomize design was adopted with 6 replicates. Treatments were; T1 = early DI (8–21 d)+latter HI (22–35 d); T2 = early DI (8–28 d)+latter HI (29–35 d), T3 = early HI (8–21 d)+latter DI (22–35 d), T4 = early HI (8–28 d)+latter DI (29–35 d) and T5 = control (white light; WT) (8–35 d) at medium intensity (20 lux). Body weight (BW), weight gain (WG), water/feed intake and ratio, feed conversion ratios (FCR) were assessed. Common behaviours (15) were recorded by scan sampling method. Lameness, foot pad dermatitis, breast blisters, hock burning damage were assessed as welfare parameters. Fear reactions were tested using Tonic Immobility Test. Ocular and carcass evaluations were done. Meat and tibiae were analyzed for fat and bone ash respectively.
Results
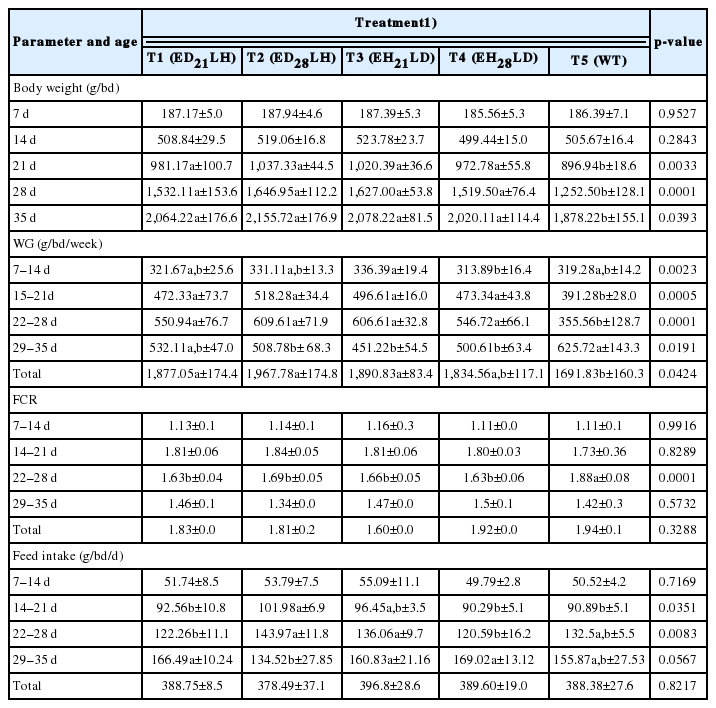
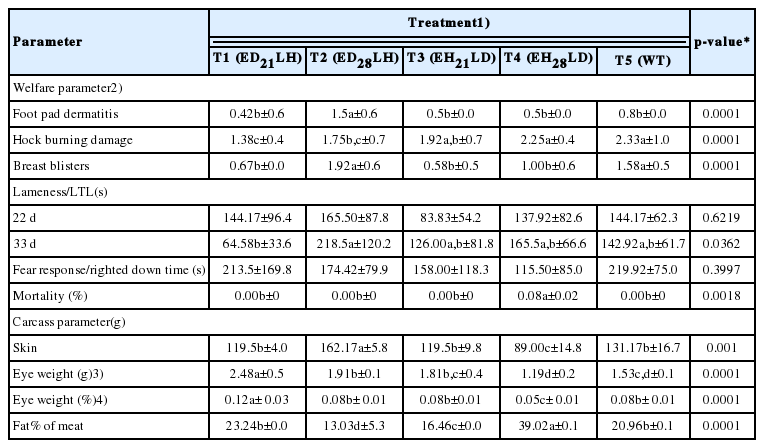
On 35 d, the highest BW (2,155.72±176 g), WG (1,967.78±174 g) were recorded by T2 compared to WT (BWWT = 1,878.22±155, WGWT = 1,691.83±160). But, application of RL, either DI, or HI during early/latter stage had no significant effect on FCR. Under HI, birds showed much higher active behaviours. DI encourages eating. Though LI changed from DI to HI, same trend could be seen even under HI. The highest leg strength (218.5±120 s) was recorded by T2. The lowest leg strength (64.58±33 s) and the highest ocular weight (2.48±1 g) were recorded by T1. Significantly (p<0.05) the highest skin weight (162.17±6 g) but the lowest fat% in meat (13.03%±5%) was recorded by T2.
Conclusion
Early exposure to DI-RL up to 28 days followed by exposure to HI-RL is the most favorable lighting regimen for optimizing production, better welfare of broilers and improving health benefits of meat.
INTRODUCTION
Light intensity (LI) manipulation is a commonly used management tool in poultry industry. It is relatively well researched with broilers having primary emphasis on production for which the impact has reported to be small or lacking. Relatively few studies have been conducted to examine the effect of LI on broiler behavior which may impinge upon welfare. Dim intensity [DI] (<10 lux) has been shown to affect bird welfare negatively as indicated by an increased incidence of skeletal disorders, foot pad health, and ocular defects [1,2]. Further, reduced activity of broilers with DI (6 lux) compared to HI (180 lux) suggested that providing HI could improve health and provide opportunities for more normal behavioral rhythms [3]. In general, LI ranging from 1 to 150 lux has been found not to affect body weight, feed consumption and feed:gain ratio [2,4].
Red light (RL) showed higher preference and improved weight gain (WG) compared to green, blue and white colour lights [5,6] in broilers.RL at DI compromised welfare status of birds and vise versa for the HI. Improved WG was recorded by DI-RL as found by [7] and [8]. Despite considerable research on general LIs, there is still a debate on the optimum level to be used for intensively housed broilers. The use of DI for commercially housed broilers is common.
We hypothesized that the birds exposed to DI light (early in life) followed by HI light (during latter part) would have resulted in much higher WG in addition to stronger legs and other welfare benefits. As exposure to unfamiliar stimuli are potentially frightening [9], we also hypothesized that birds exposed to changes of DI vs HI treatments might cause fear. The objective of the study was to investigate the most effective RL regimen using DI (5 lux) and HI (320 lux) in combination and applied at different growth stages which optimizes both production and welfare of broilers.
MATERIALS AND METHODS
Birds and housing
Research protocol has been approved (Ruh/Agri/Ethics/2012/AS02) by Research Ethics Committee of the Faculty of Agriculture, University of Ruhuna, Sri Lanka. Broiler chicks (strain Cobb, mixed sex) were brooded for 7 days (normal 40 W incandescent light at 60 lux). Paddy husk (7.5 to 10 cm) was used as litter material. On 8 d, three birds were allocated for each of the experimental units (wire mesh cages of 3′×2′ [91.44 cm×60.96 cm]) by balancing weights. All cages were provided with a feeder and a bell shaped drinker and were separated by double layered black polythene. Cages were placed in one room. The average room ambient temperature was 32°C±2°C throughout the experimental period. Variation of the ground level temperatures under HI and DI from the chick placement to the end of the experiment were ±2°C. Broiler starter (metabolizable energy [ME] = 3,000 kcal/kg, crud protein [CP] = 22%) [1–21 d]/finisher (ME = 3,100 kcal/kg, CP = 20%) [22–35 d] diets (CIC, Sri Lanka) and water provided ad libitum.
Lighting treatments
Chicks were exposed to 23 h of normal brooding light during 1 to 7 d. From 8 d onwards, birds were treated with 5 different LI treatments; T1 = (early DI [8–21 d]+latter HI [22–35 d]); T2 = (early DI [8–28 d]+latter HI [29–35 d]), T3 = (early HI [8–21 d] +latter DI [22–35 d]), T4 = (early HI [8–28 d]+latter DI [29–35 d]) and T5 = control (White light [WT]) during 8–35 d at medium intensity (MI) where HI = 320 lux, MI = 20 lux and DI = 5 lux by adopting 20L:4D schedule. Treatments were randomly assigned to 6 experimental cages by adopting complete randomize design (30 cages). Illumination was provided by 5W incandescent bulbs. LIs were recorded approximately at bird’s eye height using a digital light meter (Acklands-Grainger Inc., Ontario, Canada) and readings were averaged for each cage. Dimming of bulbs were done by dimmer switches.
Data collection
Body weight, weight gain, water/feed intake and mortality
Individual BW for each of the pen and feed/water consumption were determined on per bird basis at 7, 14, 21, 28, and 35d of age. WG and feed intake (FI) data were used to calculate feed conversion ratio (FCR). Water:feed (W:F) ratio were also determined for the relevant time periods. Experimental cages were checked for dead birds twice daily and total mortality was calculated as a percentage of initial live birds.
Behavior observation
Undisturbed behaviour of the birds was recorded for 3 consecutive hours at each 15 minutes interval in the morning (0900–1200 h), evening (1400–1700 h), and night (2200–0100 h) sessions of the day by adopting scan sampling method (4 d/wk)[10]. Behaviours were evaluated on 3 focal birds/pen and observed every week. Behaviours were recorded by one-zero measurements (presence or absence) of each behaviour. A broiler ethogram was used in diagnosing a particular behavior. Most common 15 behaviours were evaluated; lying (LY), eating at feeder (ET), drinking (DR), standing (ST), walking (WK), preening while lying (PR/LY), preening while standing (PR/ST), wing/leg stretching (WLS), dust bathing (DB), scratching floor (SF), sleeping (SL), dozing (DZ), wing flapping (WF), litter eating (LE), and other (OTH). The number of birds in each experimental unit, engaged in different activity defined by the ethogram was recorded.
Lameness assessment
Lameness was assessed using “latency to lie” test (LTL) as described by [10]. The test was performed at 2 ages; on 22 d and 33 d to test whether the LI changes made at certain points causes any effect on leg strength. Birds were placed in a water proof test pen which was flooded with a shallow layer (30 mm) of water. As chickens do not prefer to sit in water, flooding the pen motivates the birds to stand up. The time taken for each bird to lie down was recorded.
Assessment of the other welfare parameters
Contact dermatitis on the hocks (hock burning damage; HBD), footpads (foot pad dermatitis; FPD) and presence of breast blisters; BB were assessed using an internationally accepted score system [11]. HBD and BB were assessed on a 4 point scale (0, no visible damage to skin; 1, signs of skin deterioration without any redness; 2, signs of skin deterioration with presence of redness; and 3, an obvious lesion or score on any of the hocks). FPD was scored on a three point scale (0, normal footpads without lesions; 1, slight scores; 2, obvious scores on the footpads) [12]. Once each bird had been assessed, it was marked with a non toxic ink to avoid recapture. In total, 67% were assessed for each treatment for all welfare parameters.
Assessment of fearfulness by tonic immobility test at 35 d
Fear/stress reaction was tested using tonic immobility (TI) test. TI was induced by inverting the bird on its back and restrained it for 10 s in a U shaped wooden cradle as soon as a bird is caught [13]. If the bird remained immobile for 10 s after removing hands, a stopwatch was started to record latencies until the bird righted itself. If the bird righted itself in less than 10 s, then it was considered TI has not been induced and the restraint procedure was repeated. If the bird did not stay in TI more than 10 s, the same bird was retested. If a bird did not show a righting response over 10-min, retested the bird and a maximum score of 600 s was given for righting time. The observations were made in full view of the bird, about 1 m away, and eyes were fixed on the bird because of the fear-inducing properties of eye contact. One bird out of 3 from each cage (6 birds/treatment) was tested for TI.
Assessment of carcass parameters
In total, 6 birds/treatment were used for carcass evaluation. After removing the skin, each carcass was split into two halves and the internal organs (liver, gizzard, heart, and intestine) were weighed. Right leg of the each carcass (thigh+drum stick) was dissected into muscle and bones and weighed them separately.
Ocular assessment
The left and the right eyeballs of each slaughtered bird were dissected out and weighed separately within one hour after slaughtering. All the tissues were removed around the eye including optic nerve. Average eye weights were taken using a sensitive digital scale (OHAUS, Scout Pro, SP 602).
Meat analysis for crude fat
Left leg (thigh+drum stick) of carcasses was used for the analysis of fat%. Soxtec HT2 method was used to determine the fat% in meat. Meat samples were dried at 80°C until reaching a constant weight. Then the samples were ground. The extraction cups of the extraction apparatus were dried in an oven at 103°C for 1 h and cooled them in a desiccators. Ground meat samples were loaded into cellular thimbles. Each thimble was loaded with 2 g (W1) of well mixed meat sample and covered with a thin layer of cotton wool. Then the thimbles were inserted into the Soxtec system HT2 apparatus. The extraction cups were pre weighed (W2) after drying. Then 50 mL of petroleum ether was added into each of the cup. Thereafter, the cups were inserted into the Soxtec apparatus. They were kept for 15 min in the “boiling position”, for 40 min in “rinsing” position. Then the solvent was allowed to be evaporated. Then the cups were released and dried at 100°C for 30 min. Finally, the cups were cooled in desiccators until reaching a constant weight (W3). The fat% was calculated according to the formula; (Fat% = W3–W2/W1×100) (AOAC, 1990 [14]).
Analysis for tibia ash content
Right and left tibiae were analyzed for fat free ash according to [14]. Initially, tibiae were removed from the flesh and dipped in Petroleum Ether for 48 hrs. Thereafter, the samples were oven dried for 2 h. Then the dried bones were broken into 3/4” pieces and were put into a muffle furnace at 600°C to obtain ash.
Statistical analysis
Percentage behavior, performance and welfare data were first tested for normality and then statistically analyzed by using SAS. Means were separated by using least significant difference (LSD) comparisons and statistical significance was reported at p<0.05. The difference between treatment means for percentage behavior data were examined by including treatment, age, session of the day as main effects and all interactions. Lameness scores obtained by LTL; scores given for FPD, BB, and HBD were tested using Kruscal-Wallis test of the statistical package Minitab. If the interactions were significant (p<0.05), then separate analysis were done to test how the main factors alone and in combination affects on such parameters.
RESULTS AND DISCUSSION
Body weight, feed intake, and feed conversion ratio
After 14 d of treatment imposition, significantly (p<0.05) higher BWs were observed in all RL treatments over control (Table 1). Downs et al [15] reported a transient increase in BW providing 0.25 foot candle (FC), compared to 2 FC in either increasing or continuous LI programs. Similar results were observed providing 0.5 FC and 15 FC [16]. Several publications indicated increased BW in broilers provided lower LIs when comparing intensities ranging from 0.1 to 120 FC [3,17]. No previous reports have reported an interaction between age and changing LIs, as was the case in this study in which BWs were changed in relation to different intensities provided during early growing stage. However application of either HI-RL (early stage) interacted with the DI-RL (latter stage) or DI-RL (early stage) interacted with HI-RL (latter stage) had no significant effect upon final BW.
Chickens prefer to eat during the day and do not eat in darkunless LI is very low [18]. Our data also concurs with this finding. After 21 d, DI was changed in to HI in T1 while further continuing DI up to 28 d in T2 marking higher FI in T2 during 22–28 d compared to T1. After 28 d, once DI changed in to HI in T2 that resulted comparatively lower FI compared to T1 during 29–35 d (Table 1). But though DI changed into HI in T3 and T4 this trend could not be observed as this treatment also shown almost similar FI values to T1 and T2 beyond 22d. This reveled that early exposure to DI and the adaptation for increased FI under DI light would not changed though DI light changes into HI. This results showed that changes in LIs either DI to HI or HI to DI resulted proportionately marginal improvement of BW during the periods exposed to DI. In this situation the reduction in growth rate in HI can be explained by less time spend to eat. Because of this nature, finally no significant differences observed among RL intensity treatments in FI and thereby BWs. Applications of RL either DI+HI or HI+DI during early/latter stages had no significant effect on FCR. Further proving our findings FCR has been reported to be unaffected by LI as found by [15] and [16]. The lack of an effect on FCR and the parallel effects on BW and FI observed in the present study support the contention that RL intensity lighting program affects on BW are due to their influece on FI.
Effect on welfare
Occurrence of lameness, foot pad dermatitis, hock burning damage, and breast blisters
Early exposure to DI-RL followed by HI was favorable for certain welfare indices such as improved leg strength and reduced hock burns. Significantly (p<0.05) the highest FPD (1.5±1), BB (1.92±1) but reduced HBD (1.75±1) were recorded by T2. T1 which provided reduced exposure to DI light compared to T2 early in life, showed significantly (p<0.05) lower FPD and BB. This shows that lengthy exposure to DI light significantly enhances skin lesions as birds are more inactive under DI. Testing lameness at two different ages (22 d and 33 d) indicated that LI changes done at 21 and 28 days had a significant effect on leg strength as the highest leg strength was recorded by T2 at 33 day. This is the most important welfare factor to be considered as broilers could not walk properly due to increased muscle mass especially during latter part of the life [7]. This indicated that prolong exposure to DI-RL (early stage) followed by exposure to HI-RL (latter stage) resulted improved leg strength. Blatchford et al [2] found that there was no differences in gait score where 200 lux exposed broilers had more hock and foot pad bruising and fewer BB erosions than either 5 or 50 lux. They further elaborated that increased LI had little effect on broiler welfare but resulted in more pronounced behavioral rhythms. Lewis and Morris [19] found that LI did not affect performance, carcass components or the proportion of birds with leg imperfection in groups housed at 1 lux than 10 lux. These results are in line with the results of the present study. Unlike our results, the incidence of leg problems has been shown to be influenced by LI as found by [3] who did find higher frequencies of leg disorders in broilers housed in 6 lux than in 180 lux at 6 but not 3 or 8 weeks of age, although a similar experiment failed to reproduce this effect [3,17]. In an early study, [20] found that broilers actively moved towards in brighter lit (6–12 lux) than in dim lit (0.5 lux) areas and were more active in the bright areas, although there was no significant difference between constant and alternating light on leg disorders at 7 or 10 weeks of age. Furthermore, it was found that the broilers were heavier at 49 days of age when reared in 2 lux than 200 lux [21]. The authors suggested that increased activity during some crucial stage of bone development may be the cause of the increased leg health and weight differences.
Light intensity and fear response
TI was normal with no significant differences occurring between RL treatments (Table 2). Results of few studies on the influence of LI on stress responses of chickens are inconsistent. Further proving our results, [22] also found that TI and gait scores were not significantly affected by LI. Further they found that ammonia and LI did not significantly interact to TI or gait score. Campo and Davila [13] did observe that TI durations were elevated in hens exposed to 23L:1D instead of 14L:10D. Therefore, it appears that longer photoperiods and brighter intensities did not induce physiological stress responses.
Mortality
Mortality was low and unaffected by treatments. Most previous reports also indicated no effect of lower LIs on mortality [15,16], although [3] observed an increase in mortality when LI reduced from 18 to 0.6 lux. Hester et al [23] report a higher number of birds which died with signs of cannibalism in 20 than in 2.5 lux.
Carcass evaluation
Eye weight as a measure of eye morphology
Variation of LIs alone yielded significant (p<0.05) effect on eye weight. The highest and the lowest eye weights (Fresh basis) and (as % live weight basis) were recorded by T1 and T4 respectively (Table 2). This shows that early exposure to DI (up to 21 d) affects the development of the eye where prolong exposure to either DI or HI during early days in life had no effect on it. Blatchford et al [2] found that 5 lux had heavier eyes (2.33±0 g) than 50 lux (2.09±0 g) or 200 lux (2.11±0 g). Prayitno et al [7] found that LIs alone yielded no significant eye lesions. Further proving our results they found that ocular changes in the study by included significant corneal ulcerations starting at 7 days of age and the anterior chamber exhibited abnormalities at the same period. These findings were consistent with the recent report prepared by [24].
Tibia ash content
Tibia ash content was not significantly different proving that bone mineralization had no influence caused either by RD, WT color lights or different intensities of RL. Senaratna et al [8] also supports our findings as they found bone parameters were not affected by the color or intensity of light.
Skin weight and fat content in meat
Skin weight and fat% of meat showed significant difference (p<0.05) among treatments. Prolonged exposure to DI (early stage) resulted significantly higher skin weight where highest skin weight was recorded by T2. Least skin weight was recorded by HI treated birds (early stage) in T4. This is supported by other research findings where increased activity of broilers with environmental enrichment devices resulted in less thigh fat [25]. Charles et al [16] also found that DI (5 lux) resulted in increased fat and decreased protein levels of the carcass and suggested that this might be due to decreased activity in DI light. The current research has also demonstrated that birds exposed to DI (5 lux) rested more and thus supports this hypothesis. The highest and the lowest meat fat content was recorded by T4 and T2 respectively. Rearing birds in T2 is more recommendable as health benefits are in association with minimal fat content. It is suggested that lighting programs that cause a transitory decrease in growth rate is later overcome by compensatory growth, result in a delay in the progressive maturation of the growth processes. Then, although BW may catch up, this is accomplished through greater growth of normally earlier- growing carcass components such as legs and wings and a delay in the growth of normally later-growing carcass components such as breast meat.
Behavior under different red light intensity regimens
LI affected the distribution of behaviors as evidenced by multiple treatment effects and interactions. RD-LI treatment itself affected on ST, WK, and WLS. However, treatment×age×day session significantly (p<0.05) affected on LY, ET, ST, and WK behaviors. Treatment×age interaction affected (p<0.05) on LY, ET, ST, WK, and DZ whereas treatment×day session affected on ET, WK, PR/ST, DZ, and LE (Table 3). Kristensen et al [18] also supported our findings as they found broiler behavior is strongly affected by LI via affecting their visible acuity, which is varied with the age. Effects of the interactions of main factors on significant (p<0.05) behaviors are shown by Table 4. Irrespective of the time of day, significantly (p<0.05) higher ST shown by T3 during 15–21 d under HI-RL. Highest WK shown after 22 d by T1 in which the birds exposed to the shortest DI-RL early in life followed by HI-RL in latter part. This indicates provision of HI during latter part is favorable for the birds as they shown higher WK and ST. This is favorable management practice to minimize lameness condition shown by birds during the latter part of the life by increasing activities. Further proving the findings of [18], results of the current study also revealed that DI encourages ET. Though LI changed from DI to HI, effect of earlier exposure to DI is further affected to continue much higher ET behavior even during HI exposed periods. Irrespective of the day and also the treatment combination, birds show more LY under DI exposed period especially during last week.

Level of significance of the effect of different intensities of RD light treatment (TR), age (AG), and daysession (SD) and their interactions onbehavior
CONCLUSION
Birds prefer to eat under DI-RL. Though DI changed into HI, similar eating trend was recorded even during HI period. Early exposure to DI-RL up to 28 days followed by exposure to HI-RL resulted higher BW, WG, and better welfare of broilers indicated by much higher leg strength achieved by increased walking. Also the same treatment resulted less fat content in meat which associates a favorable health benefits. Therefore early exposure to DI-RL up to 28 days followed by HI-RL is the best lighting regimen in terms of optimum production, welfare of broilers and health benefits of humans.
ACKNOWLEDGMENTS
The work was financially supported by University Grants Commission, Sri Lanka (Reference No. UGC/ICD/ CRF/ CO: 3091).
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.