Quality Evaluation of Five Commercial Enzyme Linked Immunosorbent Assay Kits for Detecting Aflatoxin B1 in Feedstuffs
Article information
Abstract
The objective of this study was to evaluate the quality of five commercial enzyme linked immunosorbent assay (ELISA) kits (A, B, C, D, and E) from different suppliers for detecting aflatoxin B1 (AFB1). AFB1-free corn samples supplemented with different levels of AFB1 (5, 10, and 20 μg/kg) were used as positive controls and 6 replicates of each control sample were tested to evaluate the accuracy and precision of these kits. In addition, we also evaluated the performance of these ELISA kits for AFB1 in 30 feed samples, including corn, distillers dried grains with soluble, wheat samples, soybean meal, and poultry feed, which were verified by high performance liquid chromatography. Results showed that the coefficients of variation ranged from 1.18% to 16.22% in intra-plate and 2.85% to 18.04% in inter-plate for the determination of AFB1. The half maximal inhibitory concentration for five kits ranged from 3.72 to 7.22 μg/kg. The quantitation limits of AFB1 were all under the legal limit in China but somewhat inconsistent with kit instructions. Although the recovery rate of four of the five kits were either less than 90% or more than 110%, all these values were acceptable in practice. Two kits had high false positive rates (C and E). In conclusion, our results revealed that the qualities of five tested ELISA kits were significantly different.
INTRODUCTION
Aflatoxins (AFs) are potent, natural toxins produced by Aspergillus flavus, Aspergillus nomius, or Aspergillus parasiticus (Sweeney et al., 1998), which frequently contaminate foods and animal feedstuffs (Guan et al., 2011). In particular, feeds with high concentrations of plant materials, such as peanut, corn, soybean and rice, are more susceptible to mycotoxin contamination (Zychowski et al., 2013). Aflatoxins have caused a significant problem for the animal feed industry and been an ongoing risk to feed supply security (Bryden, 2012). AFs are of great concern because of their detrimental effects on the health of human and animals such as carcinogenic, mutagenic, teratogenic, and immunosuppressive effects (Toteja et al., 2006; Zinedine et al., 2012).
Aflatoxin B1 (AFB1), one of the most common toxins in foods and feeds, has been classified as a group 1 carcinogen by the International Agency for Research on Cancer of World Health Organization (IARC, 1993). It is harmful to human and animals because of its diverse toxicity that causes weight loss, immunosuppression, mutagenesis, reproductive alterations and carcinogenesis (Zychowski et al., 2013). Studies have showed that the prolonged feeding of gibel carp with high levels of AFB1 can inhibit reproduction activity by reducing fecundity and egg size (Huang et al., 2014); low level mycotoxin in swine and poultry diets can result in the reduction of feed intake, growth rate, egg production, carcass quality, fertility, hatchability of eggs, and immunosuppression (Harvey et al., 1991; Bryden, 2012; He et al., 2013). The ruminants fed with AFB1 contaminated feed can transfer AFB1 into milk in the form of AFs M1, which is classified as a possible human carcinogen (group 2B) (IARC, 1993).
In addition, the widespread distribution of AFB1 could be another reason for being a hazard (Binder et al., 2007). AFB1 can be found in a wide range of feedstuffs, depending on the regional differences and climate prevailing on the harvest site (Pleadin et al., 2014). It is not only the main testing items of feed hygiene standards but also the monitoring object of mycotoxins occurrence in feed and raw materials worldwide. Many surveys have showed the status of AFB1 concentrations in feedstuffs around the world (Binder et al., 2007; Streit et al., 2012; Anukul et al., 2013; Streit et al., 2013; Zachariasova et al., 2014).
Due to the frequent occurrence and toxicity of AFB1, many countries and regions have set maximum limits for AFB1. A survey shows that approximately 100 countries have developed specific limits for mycotoxins in food and feedstuffs and all countries with mycotoxin regulations have at least regulatory limits for AFB1 or the sum of AFs B1, B2, G1, and G2 in foods and/or feeds (Binder, 2007). For example, a legal limit for AFB1 in corn for animals is 50 μg/kg in China, Japan and Korea; the U.S. and Canada have set the legal limit of 20 mg/kg for total AFs (AFB1+AFB2+ AFG1+AFG2) (GB 13078–2001; Van Egmond, 2004). Details of worldwide regulations for AFB1 in food and feed are listed in a review (Van Egmond, 2004).
The low permissible limits of AFB1 require rapid, sensitive and specific analytical methods to quantify trace levels for quality control and safety assessment of feeds (Guo et al., 2014; Xie et al., 2014). Availability of immunochemical method has led to the development of many rapid and sensitive methods for monitoring and quantifying AFB1 in contaminated foods and feeds (Liu et al., 2013). Enzyme linked immunosorbent assay (ELISA) is a convenient screening tool which can provide a great saving in cost, resources, efforts and toxic solvents (Zhang et al., 2011). This method has been shown to be simple, portable, and reliable for screening a large number of samples (Li et al., 2009; Guan et al., 2011), and had become the most common rapid method for mycotoxin detection in foods and feeds (Zheng et al., 2005; Wang et al., 2011). Additionally, the detection limits of ELISA can be comparable with or even lower than those obtained by instrumental methods (Zhang et al., 2011). However, differences exist in commercial ELISA kits for the detection of AFB1. Some manufactures only launches one ELISA product for detecting AFB1 in all substance, which may reduce the performance of ELISA kits because different inferring substances co-extract from different food and feed matrixes (Li et al., 2009). Furthermore, a number of factors, such as antibodies, co-extracted compounds, the power hydrogen of the extract, and the composition of the extraction solvent, can result in over- or underestimation of AFB1 contamination. As a result, the AFB1 concentration of a same sample might be discrepant by kits from different manufacturers and even different batches of the same manufacturers. As commercialized ELISA kits are very important for monitoring AFB1 in feeds, an extensive study on the accuracy and reproducibility of different commercial ELISA kits is needed. The purpose of the present study was to evaluate the performance of five commercialized ELISA test kits for AFB1 in feed and recommend customers to keep monitoring AFB1 kits during using.
MATERIALS AND METHODS
Samples
A total of 30 feed samples that are natural contaminated by AFB1, including corn (5), distillers dried grains with soluble (5), wheat samples (5), soybean meal (5), and 10 poultry feed (5 for layer and 5 for broiler), were obtained from feed companies in China. Three batches of AFB1 ELISA kits were collected from each company. The prepared test samples were ground into a fine powder with a particle size of 1.0 mm using an analytical mill (Retsch ZM100; Haan, Germany) and stored at 4°C prior to AFB1 analysis.
Enzyme linked immunosorbent assay method
Five commercially available ELISA kits from Beijing Kwinbon Biotechnology Co., Ltd., China; Huaan Magnech Biotechnology Co., Ltd. China; Beijing Longkefangzhou Biotechnology Co., Ltd. China; ROMER International Trade (Beijing) Co., Ltd., China; and ADWK Biotechnology Co., Ltd. Beijing, China, were randomly assigned to A, B, C, D, and E. All results are consistently presented using these letters. Four of these kits were produced by domestic companies while the other one was from a foreign company. The assays were conducted following manufacturers’ instructions.
The evaluated parameters included linearity, sensitivity, accuracy, and precision. The linearity was assessed by the linear regression analysis and five levels of standard solutions in duplicates, according to respective kit instructions, were used to establish the standard curves for each plate. Commonly used methods to evaluate kit sensitivity are limit of detection (LOD) and the half maximal inhibitory concentration (IC50). The LOD was calculated from the mean value obtained with eighteen blank corn samples plus three standard deviations and the limit of quantification (LOQ) was the mean value plus ten standard deviations. The IC50, the concentration of AFB1 at 50% absorbance of zero standard solution, could be calculated from standard curve. Accuracy and precision were based on the aflatoxin recovery tests. Feed samples intended for corn with non-detectable AFB1 levels by high performance liquid chromatography (HPLC) were spiked with AFB1 at levels of 5, 10, and 20 μg/kg and then worked out recovery rate for each level. The precision of the method was determined by the coefficient of variation (CV) of intra-plate and inter-batch. The CV of intra-plate was the ratio of the standard deviation to the mean of six parallel micro wells in a same plate at each AFB1 level and the CV of inter-plate was the ratio of the standard deviation to the mean of three plates at each AFB1 level. At the same time, thirty real feed samples that the AFB1 concentrations were determined by HPLC method were used to validate the accuracy of these kits. The absorbance was measured at 450 nm in an ELISA microplate reader (power wave XS2; Bio-Tek Instruments, Burlington, Vermont, USA).
High performance liquid chromatography method
Extraction and clean-up
Aflatoxin extraction and clean-up were carried out using AFB1 Test immuno-affinity columns (Huaan Magnech Bio-Tech Co., Ltd, China), according to manufacturer’s instructions for feed samples. Five grams of each feed sample were added with 1 g NaCl and 25 mL methanol: water (70:30, v/v). The mixture was swirled for 3 min on the high speed homogenizer (Vortex-Qilinbeier 2; Scientific Industries, INC, New York, USA) and afterwards filtrated. Then, 10 mL filtrate was diluted with 20 mL ultrapure water, mixed and filtrated through micro fiber filter (Huaan Magnech Bio-Tech Co., Ltd, China). This was followed by passing 15 mL filtrate through immune affinity column. The column was eluted by 1 mL methanol and then the filtrate used for the liquid chromatography (LC) system.
HPLC analysis
A Shimadzu (LC-15C; Kyoto, Japan) LC equipped with two pumps and a RF-20A fluorescence detector were used. LC separation was performed on SB-C18 columns (150×4.6, 5 μm; Agilent, Santa Clara, CA, USA) at the flow rate of 1 mL/min and the temperature of 30°C. The mobile phase consisted of methanol and water (40/60, V/V) and the injection volume was 15 μL. Detection of AFB1 was carried out using 365 and 435 nm as wavelengths for excitation and emission, respectively. Peak areas were used for quantification.
RESULTS AND DISCUSSION
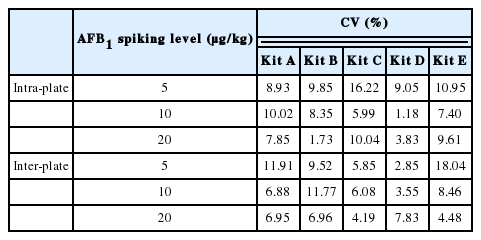
Precision
The CVs of AFB1 from five commercial kits are shown in Table 1. All CVs of intra-plate were equal or less than 10% except for kit C and E (16.22% and 10.95% respectively). The CVs of inter-batch showed that kit C and D had the best stability while kit A and E were inferior at 5 μg/kg spiked level and kit B at 10 μg/kg. The precision marked on instructions of kit A and C was less than 10% in intra-plate and inter-plate, kit D was less than 5% in intra-plate and 10% in inter-plate, kit E was less than 10% in intra-plate and 15% in inter-plate and kit B unmarked the precision on the instruction. So the data suggested that the precision of ELISA kits for AFB1 had deviation with the values marked on the instructions. However, the precision of national standard for detecting AFB1 in animal feeding should not exceed 10% (GB/T 17480-2008).
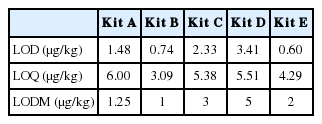
Sensitivity
The LOD and IC50 were used to evaluate the sensitivity of ELISA kit. As shown in Table 2, kit E had the lowest LOD and kit B had the lowest LOQ. The LOD marked on instructions were inconsistent with our data, which might be due to different plates with tiny diversity. Another reason could be the way we calculated LOD. In the present study, LOD was calculated from the average value obtained with eighteen blank samples plus three standard deviations. The way to determined LOD could be different. For example, others used the mean (plus 2 standard deviations) of ten blank samples (Zheng et al., 2005), a threefold confidence interval of zero analyte dose signal (Zhang, 2011) or other ways. Although the LOQ of five kits were quite different, all met the requirements of AFB1 detection in feeds that the most stringent regulation is less than 10 μg/kg in China (Guo et al., 2014). Early studies showed that LOD of AFB1 was 1 μg/kg in maize using direct competitive ELISA (Karami-Osboo et al., 2012). Rossi (Rossi et al., 2012) reported that LOQs in broiler feed and laying hen feed were 1.43 and 1.75 μg/kg using indirect competitive ELISA (ic-ELISA) based on an anti-AFB1 monoclonal antibody, respectively. The ELISA instructions indicated that A, B, and E used ic-ELISA while C and E used direct competitive ELISA. For the LODs of five kits, our data support the view that ci-ELISA is more sensitive than the competitive direct ELISA (Zhang et al., 2011). Matrix materials also had great effects on results because of the different inferring substances co-extracted from different food and feed matrixes (Lee et al., 2004). In this study, four of the five kits apply to cereals, grains as raw material in food and feed and meat while one (kit C) is exclusively used in feed. These results demonstrated that antibody type, reaction type, and matrix might have effects on the ELISA test kits for LOQ or LOD. All these factors may explain variances in sensitivity of these kits.
IC50 was another index for sensitivity, which was calculated through the standard curve. The IC50 was the average of three batches ranged from 3.72 to 7.22 μg/kg and kit D had the lowest value (Table 3). The CVs of IC50 of all kits were different, which might reflect the difference of inter-plates of five kits.
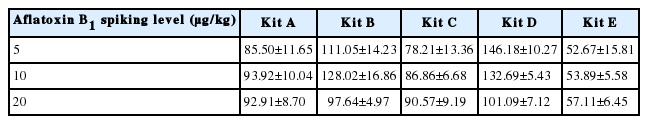
Accuracy
A recovery study was performed out to test the accuracy of the five ELISA kits for the determination of AFB1 in AFB1-free corn samples and samples spiked with different concentration of AFB1 (5, 10, and 20 μg/kg). Excellent recovery rates were found for kit A from fortified samples at the level of 5 to 20 μg/kg, ranging from 85.50% to 93.92% (Table 4), which were much closer to 100% than any other kits. Compared with kit A, kit C has a lower recovery rates at 5 μg/kg. Literatures showed that the recoveries of spiked rice samples with 10 to 500 μg/kg AFB1 were from 94% to 113% using direct competitive ELISA using a monoclonal antibody (Kolosova et al., 2006). According to the Commission of the European Communities (2006), the critical values for recovery rates of AFB1 are 70% to 110% for concentrations between 1 and 10 μg/kg and 80% to 110% for concentrations higher than 10 μg/kg. Therefore, only kit A and C met the recommended standards. Although the recovery rates of other kits were either less than 80% or more than 110%, all the values were acceptable in practice.
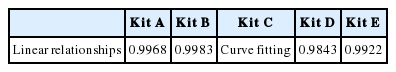
Linearity
The linearity was assessed according to the linear regression analysis with five levels of standard solutions in duplicate. Standard curve was drawn using a mathematical model of log (dose)-logit(B/B0):
The standard curves of kit A, B, and E were linear relationships while that of kit C was curvilinear (Table 5). It is generally believed that linear relationship is better than curvilinear relationship.
Analysis of feed samples
The concentrations of 30 feed samples for AFB1 were determined by ELISA kits and HPLC was used as a reference method. The calibration curve of HPLC method for AFB1 was constructed with standards of 0, 0.625, 1.25, 2.5, 5, and 10 μg/kg with the LOD of 0.15 μg/kg and LOQ of 0.5 μg/kg. The correlation coefficient of standard curve was 0.992. The retention time of AFB1 was about 9.9 min. The average recovery rate was 108.7% at 10 μg/kg AFB1 spiking in corn samples and 104.3% at 20 μg/kg.
The abilities to detect AFB1 of five commercial kits were evaluated by feed samples in current study. The AFB1-concentrations of 30 feed samples were under 0.5 μg/kg except for two samples (3.2 μg/kg and 13.5 μg/kg, respectively), which could not be representative in the linear range of ELISA kits. However, the results reflected the abilities of screening samples that concentrations of AFB1 were below LODs to some extent. Therefore, we selected respective LODs marked on instructions as judgment standards. Our results shown in Table 6 indicated that the five commercial kits exhibited great differences in accuracy, and kit A and D performed better than others and kit D had the highest LOD. A survey evaluated the mycotoxin contamination in feed and feed raw materials worldwide using HPLC method in particular for more complex matrices which would interfere with the ELISA method, such as DDGS, finished feed, silage, or straw. Only single commodities such as maize or wheat were analysed with ELISA (Streit et al., 2013). Matrix effect is especially obvious in cases of high complexity of the test material, which can lead to overestimates, underestimates, or even false negative or false positive results (Binder, 2007). As a general rule, ELISA methods allow a certain degree of false positive rate because positive samples will be confirmed again by HPLC or other analysis methods. When the concentration of AFB1 in a sample exceeds the legal limit, all kits could detect correctly.
CONCLUSION
The present study confirmed that significant variations in sensitivity and accuracy for AFB1 detection existed among the 5 commercial ELISA kits. It can be concluded that kit A has good linear regression and correct rate; kit B has good linear regression and low correct rate; kit C and E showed low correct rate with high false positive rate, and kit D performed good accuracy under a highest LOD of them. Besides, kits products from different manufactures had great differences in CVs of intra-plate and inter-plate. Therefore, all parameters used for quality evaluation which had mentioned above should be considered comprehensively. For AFB1 detection, early studies showed that detection limits of ELISA methods are comparable to HPLC (Anklam et al., 2002) and that is one of most important reasons why ELISA kits are widely used. Although many commercial ELISA kits are now available for the detection of AFB1, their quality remarkably differs.
In conclusion, the present study showed that there are significant differences in qualities among the ELISA kits for AFB1 detection. Our data emphasized the necessity to regulate the market for AFB1 ELISA kits and reminded users to do some assessments before using in order to obtain more reliable data. It is also important for the regulatory authorities to keep monitoring these products to ensure consumer rights and feed safety.
ACKNOWLEDGMENTS
The authors are very grateful to Special Fund Project for Beijing Poultry Industry Innovation Team in Modern Agricultural Technology System (Serial number, C21108) and Special Fund for Agro-scientific Research in the Public Interest (Serial number, 20120323).