Cellular Uptake and Cytotoxicity of β-Lactoglobulin Nanoparticles: The Effects of Particle Size and Surface Charge
Article information
Abstract
It is necessary to understand the cellular uptake and cytotoxicity of food-grade delivery systems, such as β-lactoglobulin (β-lg) nanoparticles, for the application of bioactive compounds to functional foods. The objectives of this study were to investigate the relationships between the physicochemical properties of β-lg nanoparticles, such as particle size and zeta-potential value, and their cellular uptakes and cytotoxicity in Caco-2 cells. Physicochemical properties of β-lg nanoparticles were evaluated using particle size analyzer. Flow cytometry and confocal laser scanning microscopy were used to investigate cellular uptake and cytotoxicity of β-lg nanoparticles. The β-lg nanoparticles with various particle sizes (98 to 192 nm) and zeta-potential values (−14.8 to −17.6 mV) were successfully formed. A decrease in heating temperature from 70°C to 60°C resulted in a decrease in the particle size and an increase in the zeta-potential value of β-lg nanoparticles. Non-cytotoxicity was observed in Caco-2 cells treated with β-lg nanoparticles. There was an increase in cellular uptake of β-lg nanoparticles with a decrease in particle size and an increase in zeta-potential value. Cellular uptake β-lg nanoparticles was negatively correlated with particle size and positively correlated with zeta-potential value. Therefore, these results suggest that the particle size and zeta-potential value of β-lg nanoparticles play an important role in the cellular uptake. The β-lg nanoparticles can be used as a delivery system in foods due to its high cellular uptake and non-cytotoxicity.
INTRODUCTION
Bioactive compounds, such as plant polyphenols and carotenoids, have been used in functional foods providing many health benefits since they may enhance antioxidant, anticancer, and antiviral activities and decrease the risks of diseases (Chen and Subirade, 2005; Chen et al., 2006; Hu et al., 2012). However, these bioactive compounds often have poor bioavailability due to their low aqueous solubility, low permeability across the intestinal cells, and low stability during food processing and storage (Chen and Subirade, 2005; Chen et al., 2006; Hu et al., 2012). To overcome these problems, various delivery systems have been developed to entrap, protect, and deliver bioactive compounds to small intestine enhancing the bioavailability of compounds (Chen and Subirade, 2005; Chen et al., 2006; Zimet and Livney, 2009; Kumari and Yadav, 2011). Although many delivery systems have been successfully made using synthetic polymers, the production of synthetic polymer-based delivery systems may need the use of organic solvent and relatively hasher formulation condition. Therefore, food-grade materials should be used for the manufacture of delivery systems for food applications (Chen and Subirade, 2005; Chen et al., 2006). In this study, beta-lactoglobulin (β-lg), the major component of whey proteins, was selected as a food-grade delivery material. The β-lg has great potentials for the preparation of delivery systems, which is applicable to foods due to its generally regarded as safe status, high nutritional level, biodegradable nature and gel forming capability (Chen and subirade, 2005; Livney, 2010).
In our previous study, modulating manufacturing variables including heat treatment resulted in the formation of whey protein nano delivery systems with various sizes and surface charges (Lee et al., 2008; Ha et al., 2013; Lee et al., 2013). It is known that reducing the particle size of delivery systems may enhance the bioavailability of bioactive compounds due to an increase in gastrointestinal transit time, adhesion to and interaction with biological cells, and cellular uptake of delivery systems (Win and Feng, 2005; Zhang et al., 2008; Iversen et al., 2011; Kumari and Yadav, 2011). Moreover, a decrease in the particle size from macroscopic scale to nanoscopic leads to changes in physico-chemical properties of the delivery materials and thus particles with smaller size may elicit different biological responses including toxicity (Yin et al., 2005; Napierska et al., 2009; Powell et al., 2010). On the other hand, the surface charge of nanoparticles can be considered as an important factor to affect the cellular uptake of nanoparticles because of the electrostatic interactions between the surface of nanoparticles and charged cell surface (Mansouri et al., 2006; Cho et al., 2009; Verma and Stellacci, 2010). Although the cellular uptake and cytotoxicity of several food-grade nanoparticles, such as chitosan-based (Zhang et al., 2008) and chitosan-caseinophosphopeptide (Hu et al., 2012) nanoparticles has been examined, there is a lack of knowledge on the delivery of food-grade nanoparticles, especially β-lg nanoparticles, to intestinal cells and cytotoxicity. Therefore, more studies on the cellular uptake and cytotoxicity of β-lg nanoparticles are needed to apply β-lg nanoparticles with bioactive compounds to functional foods. In this study, we hypothesize that the particle size and surface charges of β-lg nanoparticles modulated by heat treatments can be a major factor to affect their cellular uptakes in Caco-2 cells.
The objectives of this study were to manufacture β-lg nanoparticles with various sizes and surface charges by changing heating temperature, to determine the relationships between the particle size and surface charge of β-lg nanoparticles and their cellular uptakes in Caco-2 cells, and to evaluate the cytotoxicity of β-lg nanoparticles.
MATERIALS AND METHODS
Chemicals and reagents
The β-lg was generously supplied by Davisco International, Inc. (Le Sueur, MN, USA). Calcium chloride (CaCl2), fluorescein isothiocyanate (FITC), and propidium iodide (PI) were purchased from Sigma-Aldirich Inc. (St. Louis, MO, USA). Sodium hydroxide (NaOH) was purchased from Yakuri Pure Chemicals Co. (Osaka, Japan).
Preparation of fluorescein isothiocynate-conjugated beta-lactoglobulin nanoparticles
The FITC-conjugated β-lg nanoparticles were manufactured by the internal gelation method of Ha et al. (2013) and Hu et al. (2012). The β-lg solutions (1%, w/v) were treated with 4 mM CaCl2 and adjusted to pH 9.5 using 0.1 M NaOH. To investigate the cellular uptake of β-lg nanoparticles, FITC, a fluorescent marker, was used (Hu et al., 2012). To manufacture FITC-conjugated β-lg, 0.1 mL of FITC solution (40 mg/mL in ethanol) was added to 10 mL of β-lg solution in 0.1 M carbonate buffer at pH 9.0 and then FITC/β-lg mixtures reacted for 2 h in a dark room. To remove unreacted FITC, FITC/β-lg mixtures were dialyzed against distilled water for 48 h using a dialysis membrane (3.5 kDa molecular weight cut-off, Thermo Scientific, Rockford, USA) in a dark room. Those dialyzed mixtures were freeze dried to from FITC-conjugated β-lg. To manufacture FITC-conjugated β-lg nanoparticles, 1% (w/v) FITC-conjugated β-lg solutions were treated with 4 mM CaCl2 and adjusted to pH 9.5 using 0.1 M NaOH. The FITC-conjugated β-lg solutions were heated at 60°C, 65°C, or 70°C for 10 min using a water bath (Wisecircu, Daihan Scientific, Inc., Seoul, Korea) and then cooled to room temperature in ice water.
Particle size and zeta-potential measurements
The particle size (mean particle diameter, Z-average) and zeta-potential value of β-lg nanoparticles were assessed by the use of Zetasizer Nano ZS (Malvern Instruments, UK). Nanoparticles were diluted (1:10) with deionized water prior to analysis. The particle size and zeta-potential value of nanoparticles were measured at room temperature with scattering angles of 90° and 20°, respectively.
Cell culture condition
Caco-2 cells, which are human epithelial colorectal adenocarcinoma cells, were used as in vitro model to investigate the cellular uptake and cytotoxicity of β-lg nanoparticles. Caco-2 cells (ATCC HTB37) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Caco-2 cells were cultured in Dulbecco-Vogt modified Eagle medium (DMEM, Lonza BioWhittaker, Walkersvile, MD, USA) including 20% fetal bovine serum and 1% penicillin-streptomycin and maintained in 5% CO2 incubator at 37°C.
Cytotoxicity and cellular uptake of beta-lactoglobulin nanoparticles: Quantitative studies
Caco-2 cells were seeded in 6-well plates and incubated at 37°C, 5% CO2 in CO2 incubator for 24 h to form a confluent monolayer. Culture medium was replaced by serum-free DMEM and incubated at 37°C for 30 min. FITC-conjugated β-lg nanoparticles dissolved in serum-free DMEM were added to Caco-2 cells and then incubated at 37°C for 0.5, 1, 2, 3, or 4 h to initiate the cellular uptake of nanoparticles. Final concentration of β-lg nanoparticles in serum-free DMEM was 100, 250, or 500 μg/mL. To remove free nanoparticles in serum-free DMEM, cells were washed three times with phosphate-buffered saline (PBS, pH 7.4) and collected with trypsin treatment. The FITC-conjugated β-lg nanoparticles were used as a marker to quantify the cellular uptake of β-lg nanoparticles. Cytotoxicity and cellular uptake of nanoparticles were evaluated by FACSCalibur flow cytometry and CellQuest Pro software (Becton Dickenson, Mississauga, CA, USA). In nanoparticle treated cells, cell viability was evaluated with PI staining followed by flow cytometric analysis (Russell et al., 2004; Chen et al., 2009). Excitation and emission wavelengths were 488 nm and 590 nm, respectively. The percentage of live and dead cells was determined by measuring the PI-negative cells and PI-positive cells, respectively. The cell survival ratio was expressed as percent of PI-negative cells per control cells which were cultured in nanoparticle-free medium. The cellular uptake of FITC-conjugated β-lg nanoparticles in Caco-2 cells was also analyzed using flow cytometry at 488 nm excitation wavelength and 530 nm emission wavelength (Pushpanathan et al., 2012). The FITC-positive cells indicate the number of Caco-2 cells that have uptaken β-lg nanoparticles. Cellular uptake efficiency was expressed as percent of FITC-positive cells per total cells.
Cellular uptake of beta-lactoglobulin nanoparticles: Qualitative studies
Confocal laser scanning microscopy (CLSM) was used to observe the cellular uptake of FITC-conjugated β-lg nanoparticle in Caco-2 cells. To observe Caco-2 cells, cells were grown on a glass microscope cover-slip. The nuclei of Caco-2 cells were stained with PI (0.1 mg/mL). Cover-slips were then inverted and mounted onto microscope slides and cellular uptake of nanoparticles were observed using a CLSM (FV1000, Olympus, Tokyo, Japan) equipped with a ×40 (numerical aperture, 1.0) oil immersion lens. Fluorescent images were analyzed using a confocal microscopy (Fluoview FV1000, Olympus, Tokyo, Japan). Fluorescence signals were detected at excitation/emission wavelengths of 535/617 nm (PI, red) and 488/530 nm (FITC, green), respectively.
Statistical analysis
All data were expressed as mean±standard errors. The significance of the effects of FITC conjugation, heating temperature, and their interaction was analyzed by a two-way analysis of variance (ANOVA). One-way ANOVA with the Fisher’s least significant difference (LSD) test was used to analyze the effects of heating temperature on the zeta-potential value, cytotoxicity, and cellular uptake of β-lg nanoparticles. Pearson’s correlation coefficient analysis was used to determine the relationships between dependent variables (heating temperature, particle size, and zeta-potential of β-lg nanoparticles). The level of significance was set at the 5% level (p<0.05). All statistical analyses were used with SAS software package (2003).
RESULTS AND DISCUSSION
Physicochemical properties of fluorescein isothiocyanate- conjugated beta-lactoglobulin nanoparticles
Effects of heating temperature on the physicochemical properties of β-lg nanoparticles, such as particle size and zeta-potential, were shown in Figures 1 and 2. An increase in heating temperature from 60°C to 70°C resulted in a significant (p<0.05) increase in the size of β-lg nanoparticles from 97 to 176 nm (Figure 1). It was reported that the heat treatment of β-lg from 60°C to 70°C may lead to increased surface hydrophobicity and free sulfhydryl groups exposed to aqueous environment determined by ANS probe method and DTNB method, respectively (Lee et al., 2008; Lee et al., 2013). An increase in surface hydrophobicity and exposed free sulfhydryl residues can be a driving force to increase intermolecular hydrophobic attractions and thiol-disulfide interactions, which may lead an increase in the size of β-lg nanoparticles. Similar results were reported that the size of whey protein particles was increased from 70 to 200 nm as heating temperature was increased from 58°C to 77°C (Gracia-Julia et al., 2008). Conjugation of FITC with β-lg did not significantly (p<0.05) affect the size of nanoparticles (Figure 1).
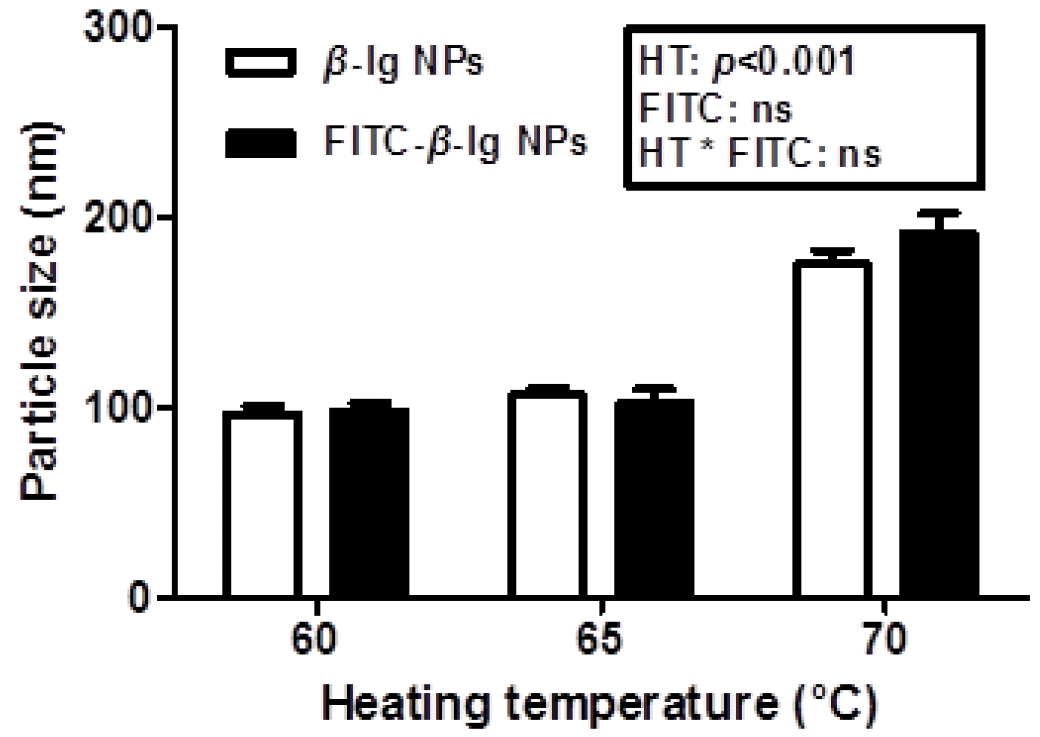
Particle size of β-lactoglobulin (β-lg) and fluorescein isothiocyanate-conjugated β-lg nanoparticles prepared at various heating temperature (60°C, 65°C, and 70°C). NPs, nanoparticles; HT, heating temperature; ns, not significant (p>0.05).
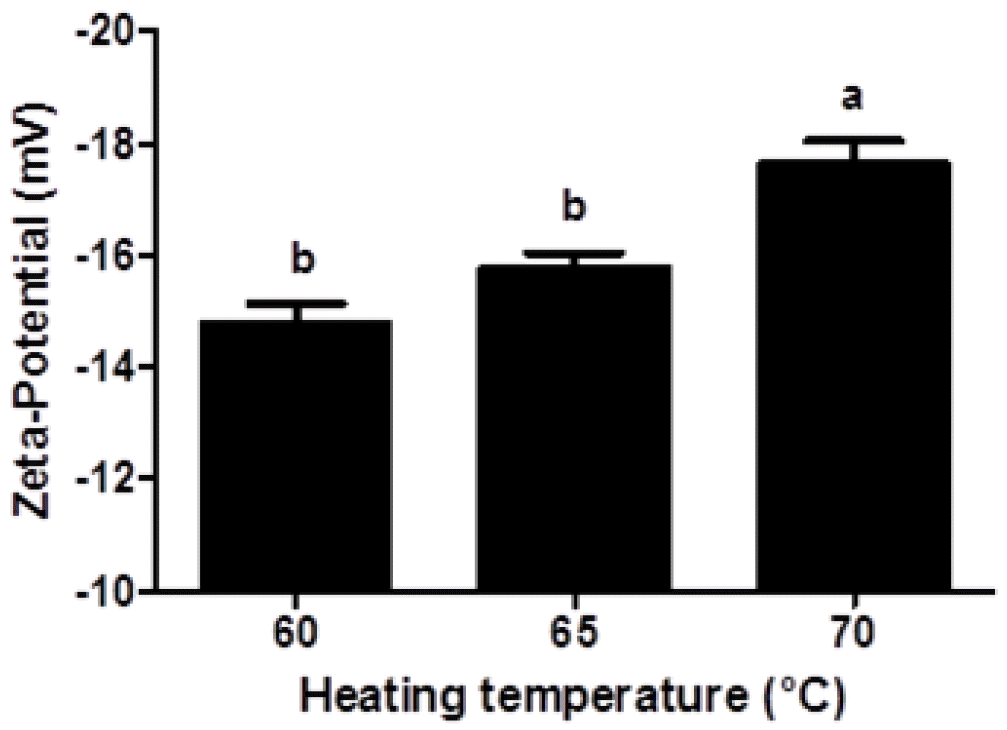
Zeta-potential of fluorescein isothiocyanate-conjugated β-lactoglobulin nanoparticles prepared at various heating temperature (60°C, 65°C, and 70°C). Different letters on a column indicate significant differences (p<0.05).
Negative zeta-potential values (−14.8 to −17.6 mV) were observed in β-lg nanoparticles, which indicates that β-lg nanoparticle had negative surface charges. Since the isoelectric point of β-lg is about 5.3, β-lg nanoparticles manufactured at pH 9.5 had negative surface charges. Zeta-potential value of β-lg nanoparticles was significantly (p<0.05) decreased from −14.8 to −17.6 mV when heating temperature was increased from 60°C to 70°C (Figure 2). An increase in heating temperature may lead to partial unfolding of β-lg. And thus, negatively charged amino acids buried inside of β-lg could be exposed to the surface of β-lg, which could result in a decrease in the zeta-potential value of β-lg nanoparticles. Similar results were observed for β-lg dispersions heated at 85°C for 10 min (Schmitt et al., 2009).
Cytotoxicity of beta-lactoglobulin nanoparticles
There were growing interests in the safety and toxicity of nanoparticles since the small size of nanoparticles may cause toxicity problem (Yin et al., 2005; Napierska et al., 2009). A reduction in the size of nanoparticle led to an increase in the surface area, which can enhance interactions between nanoparticles and cell membranes (Zhang et al., 2008). Interactions between charged nanoparticles and cell membranes may induce the transient poration of cell membranes, which can be related to the cytotoxicity (Cockburn et al., 2012). The cytotoxicity of β-lg nanoparticles in Caco-2 cells were determined by measuring the cell survival ratio using flow cytometry prior to cellular uptake study (Figure 3). The measurement of cell survival ratio is the common method to investigate the in vitro cytotoxicity of biomaterials (Hu et al., 2012). Nanoparticle concentration (100 to 500 μg/mL) and incubation time (0.5 to 4 h) did not significantly (p<0.05) affect the cell survival ratio. When Caco-2 cells were treated with FITC-conjugated β-lg nanoparticles at concentration of 250 μg/mL for 2 h, no significant differences in cell survival ratio were observed indicating that β-lg nanoparticles had no cytotoxicity to Caco-2 cells (Figure 3). Although β-lg nanoparticles had very small size ranging from 98 to 192 nm, the size of β-lg nanoparticles was not an important determinant in cytotoxicity. Similar results were reported for food-grade nanoparticles. Zhang et al. (2008) reported that oleyl-chitosan nanoparticles exhibited no cytotoxicity when nanoparticles were treated with concentration up to 400 μg/mL on A549 cells. Chitosan-caseinophosphopeptide nanoparticles with concentration below 500 μg/mL were also non-toxic to Caco-2 cells (Hu et al., 2012).
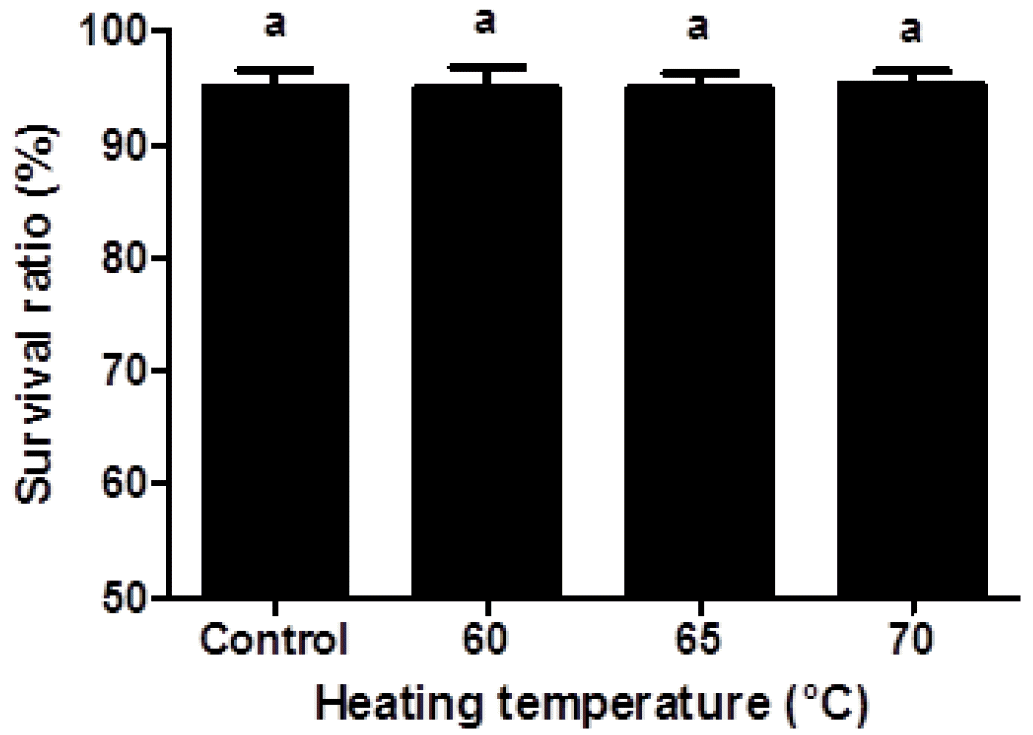
Viability of Caco-2 cells treated with β-lactoglobulin (β-lg) nanoparticles prepared at various heating temperature (60°C, 65°C, and 70°C). The β-lg nanoparticles (250 μg/mL) were added to Caco-2 cells and incubated at 37°C for 2 h. Different letters on a column indicate significant differences (p<0.05).
Cellular uptake of beta-lactoglobulin nanoparticles: Quantitative studies
The in vitro or in vivo cellular uptake of nanoparticles was investigated by the use of fluorescent-based method (Win and Feng, 2005). In this study, Caco-2 cells were selected as in vitro model to investigate the cellular uptake of β-lg nanoparticles in small intestine since there are similar morphological and functional properties between Caco-2 cell lines and small intestinal enterocytes (Win and Feng, 2005; Powell et al., 2010). Fluorescein isothiocynate, was used as a fluorescent marker to determine the cellular uptake of β-lg nanoparticles in Caco-2 cells (Hu et al., 2012). Since the concentration and incubation time of nanoparticle could be key factors to affect the in vitro cellular uptake of nanoparticles (Zhang et al., 2008), the effects of concentration and incubation time of nanoparticles on the cellular uptake of β-lg nanoparticles were examined (Figure 4). As the concentration of β-lg nanoparticles was increased from 100 to 250 μg/mL, the cellular uptake of β-lg nanoparticles was significantly (p<0.05) increased (Figure 4A). However, a further increase in the concentration of nanoparticles from 250 to 500 μg/mL did not significantly (p<0.05) affect the cellular uptake of nanoparticles indicating that a saturation limit of nanoparticle was 250 μg/mL. An increase in incubation time from 0.5 to 2 h resulted in a significant (p<0.05) increase in the cellular uptake of β-lg nanoparticles while no significant (p<0.05) differences in the cellular uptake of β-lg nanoparticles after 2 h incubation were observed (Figure 4B). Therefore, FITC-conjugated β-lg nanoparticle suspensions with 250 μg/mL concentration and 2 h incubation time were used for further experiments.

Effects of nanoparticle concentration and incubation time on the cellular uptake of β-lactoglobulin (β-lg) nanoparticles in Caco-2 cells. (A) Various concentration of fluorescein isothiocyanate (FITC)-conjugated β-lg nanoparticles (100, 250, and 500 μg/mL) were added to Caco-2 cells and incubated at 37°C for 2 h. (B) FITC-conjugated β-lg nanoparticles (250 μg/mL) were added to Caco-2 cells and incubated at 37°C for 0, 0.5, 1, 2, 3, 4 h. Different letters on a column indicate significant differences (p<0.05).
It is believed that the cellular uptake of nanoparticles was affected by various morphological- and physicochemical properties of nanoparticles, such as their size and surface charge (Mansouri et al., 2006; Kumari and Yadav, 2011; Yoo et al., 2011). Effect of heating temperature on the cellular uptake of β-lg nanoparticles was displayed in Figure 5. A decrease in heating temperature from 70°C to 60°C led to a decrease in the size of β-lg nanoparticles from 192 to 98 nm (Figure 1). The cellular uptake of β-lg nanoparticles was significantly (p<0.05) increased as heating temperature decreased from 70°C to 60°C (Figure 5). Smaller β-lg nanoparticles prepared at lower heating temperature had higher uptake efficiency of Caco-2 cell compared to larger β-lg nanoparticles treated at higher heating temperature. There were significantly negative correlations between the particle size and the cellular uptake of β-lg nanoparticles with r values of −0.76 (p<0.001) (Table 1). Since nanoparticles with smaller size had larger surface areas available to adhere and interact with cell membranes, a decrease in the size of nanoparticles may lead to an increase in the interactions and binding with cell membranes, which can result in enhanced cellular uptake of nanoparticles (Win and Feng, 2005; Chen et al., 2006; Zhang et al., 2008; Kumari and Yadav, 2011; Iversen et al., 2011; Ha et al., 2013). Similar results were reported for oleyl chitosan and PLGA nanoparticles (Win and Feng, 2005; Zhang et al., 2008). The cellular uptake of oleyl chitosan nanoparticles was increased from 7.6% to 8.8% when the size of nanoparticle was decreased from 307 to 199 nm (Zhang et al., 2008). Win and Feng (2005) reported that a reduction in the size of PLGA nanoparticles from 1,000 to 100 nm resulted in an increase in the cellular uptake of nanoparticles from 9% to 14% in Caco-2 cells.
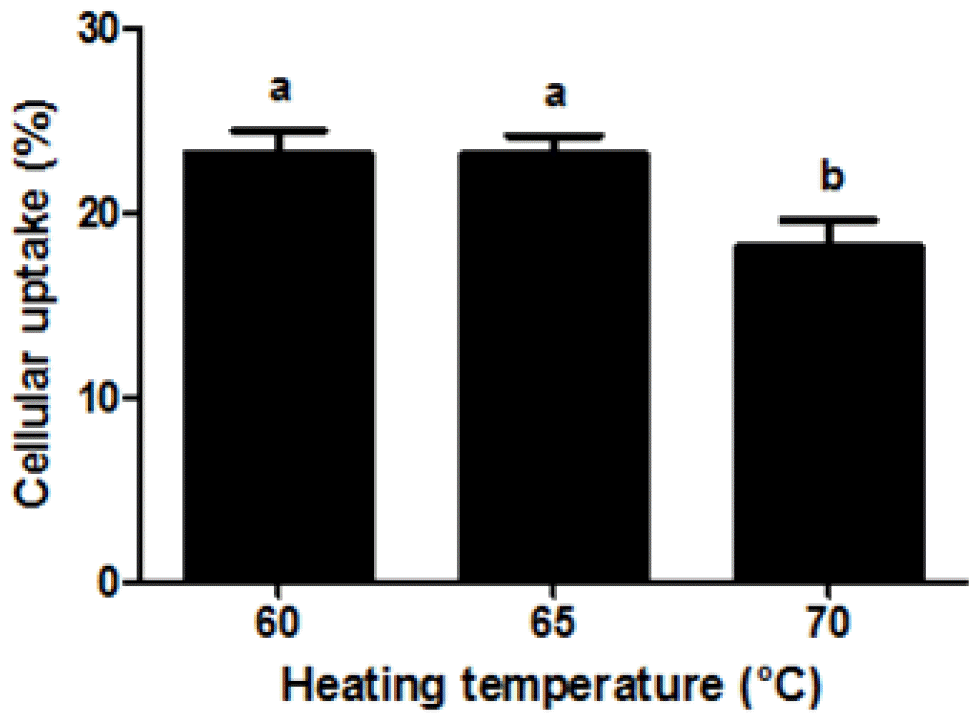
Effect of heating temperature on the cellular uptake of fluorescein isothiocyanate (FITC)-conjugated β-lactoglobulin (β-lg) nanoparticles in Caco-2 cells. FITC-conjugated β-lg nanoparticles (250 μg/mL) were added to Caco-2 cells and incubated at 37°C for 2 h. Different letters on a column indicate significant differences (p<0.05).
On the other hand, an increase in the cellular uptake of β-lg nanoparticles was observed when β-lg nanoparticles treated at lower heating temperature had higher zeta-potential value (surface charge) (Figure 5). Cellular uptake of β-lg nanoparticles was positively correlated with zeta-potential of β-lg nanoparticles (r = 0.75, p<0.01) (Table 1). Similar results was reported by He et al. (2010), who found that an increase in zeta-potential values of carboxymethyl chitosan grafted methyl methacrylate nanoparticles from −40 to −15 mV resulted in a significant increase in the cellular uptake of nanoparticles in murine macrophage cells. An increase in heating temperature may lead to an increase in the zeta-potential value of β-lg nanoparticles, which could result in stronger electrostatic repulsion between the β-lg nanoparticles and the cell membranes (He et al., 2010). This may cause a reduction in the adhesion and interaction of β-lg nanoparticles with Caco-2 cell surface and hence a decrease in cellular uptake of nanoparticles.
Cellular uptake of beta-lactoglobulin nanoparticles: Qualitative studies
Qualitative study on the cellular uptake of FITC-conjugated β-lg nanoparticles were carried out using a CLSM. The cellular uptake of β-lg nanoparticles treated at various heating temperatures was shown in Figure 6. Propidium iodide (red fluorescence) was used to visualize the nuclei of Caco-2 cells while FITC (green fluorescence) was conjugated to β-lg to observe the cellular uptake of nanoparticles. Confocal microscopic images of Caco-2 cells treated with FITC-conjugated β-lg nanoparticles were examined with PI channel (Figure 6, left panel), FITC channel (Figure 6, middle panel), and combined PI channel and FITC channel (Figure 6, right panel). The nuclei of Caco-2 cells were stained with PI (red) and displayed in Figure 6 (left panel). In Figure 6 (middle panel), the green color exhibited FITC-conjugated β-lg nanoparticles prepared at 60°C, 65°C, or 70°C heating temperature. The overlaying images of Caco-2 cell nuclei (PI, red) and β-lg nanoparticles (FITC, green) were shown in Figure 6 (right panel). In Figure 6 (right panel), β-lg nanoparticles were observed around the nuclei of Caco-2 cells (in the cell membrane and inside the cytosol) indicating that β-lg nanoparticles were successfully taken up by Caco-2 cells. No fluorescence was observed in the confocal microscopic image of control cells (data not shown) indicating that Caco-2 cell or β-lg do not have autofluorescence.
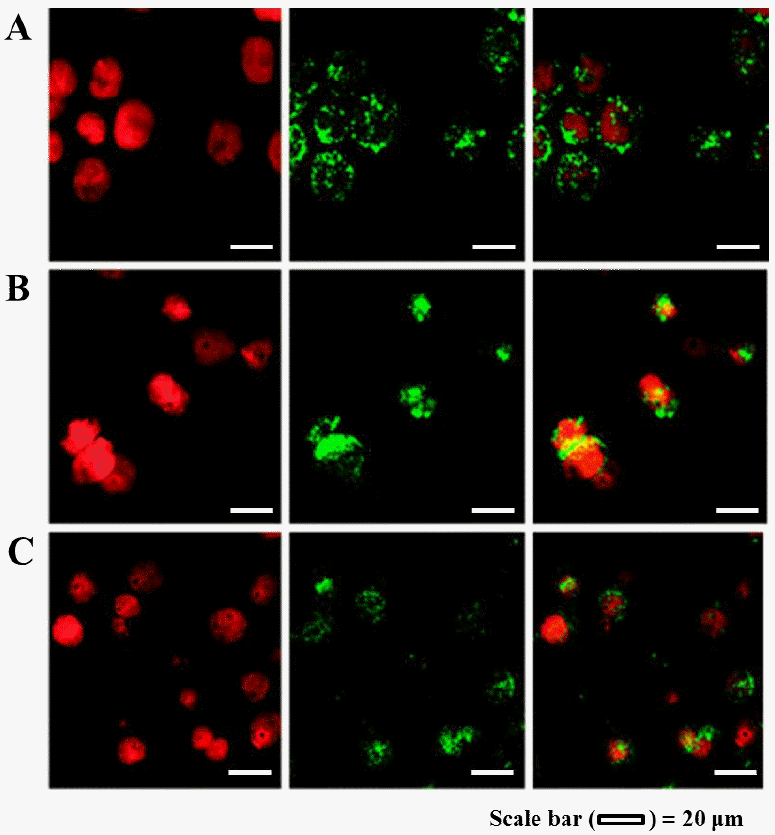
Confocal laser scanning microscopy images of Caco-2 cells after 2 h incubation at 37°C with fluorescein isothiocyanate (FITC)-conjugated β-lactoglobulin (β-lg) nanoparticles (250 μg/mL) prepared at (A) 60°C, (B) 65°C, and (C) 70°C heating temperatures. To observe the cellular uptake of β-lg nanoparticles, cells were stained with propidium iodide (PI) and FITC-conjugated β-lg nanoparticles were used. Cellular uptake of FITC-conjugated β-lg nanoparticles was imaged by PI channel (left panel), FITC channel (middle panel), and combined PI channel and FITC channel (right panel), respectively.
In conclusion, food-grade β-lg nanoparticles with various particle size and surface charge were successfully manufactured by the internal gelation method. The β-lg nanoparticles were non-cytotoxic to Caco-2 cells and can be taken up by Caco-2 cells. Heating temperature can modulate the size and surface charge of β-lg nanoparticles. The size and surface charge of β-lg nanoparticles were crucial factors affecting the cellular uptake of β-lg nanoparticles in Caco-2 cells. A better understanding of the factors contributing to the cellular uptake of nanoparticles could allow dairy processors to apply β-lg nanoparticlesas a delivery system in functional foods.
ACKNOWLEDGMENTS
This research was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (PJ009659032013)” Rural Development Administration and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0013978; 2012R1A1A4A01013793), Republic of Korea.
