Recombinant Goat VEGF164 Increases Hair Growth by Painting Process on the Skin of Shaved Mouse
Article information
Abstract
To detect goat vascular endothelial growth factor (VEGF)-mediated regrowth of hair, full-length VEGF164 cDNA was cloned from Inner Mongolia cashmere goat (Capra hircus) into the pET-his prokaryotic expression vector, and the recombinant plasmid was transferred into E. coli BL21 cells. The expression of recombinant 6×his-gVEGF164 protein was induced by 0.5 mM isopropyl thio-β-D-galactoside at 32°C. Recombinant goat VEGF164 (rgVEGF164) was purified and identi ed by western blot using monoclonal anti-his and anti-VEGF antibodies. The rgVEGF164 was smeared onto the dorsal area of a shaved mouse, and we noted that hair regrowth in this area was faster than in the control group. Thus, rgVEGF164 increases hair growth in mice.
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a specific heparin-binding glycoprotein (Ferrara and Henzel, 1989) that is also known as vascular permeability factor (VPF) (Senger et al., 1993). The VEGF is a homodimer that comprises 2 polypeptide chains with a molecular mass of approximately 45 kDa (Ferrara and Henzel, 1989; Gospodarowicz et al., 1989). The human VEGF gene has 8 exons that are separated by 7 introns. By alternative splicing, 7 VEGF transcripts are expressed in human, encoding polypeptides of 189, 165, 121, 145, 183, 148, and 206 amino acids, respectively (Jingjing et al., 1999; Poltorak et al., 1997; Tischer et al., 1991; Whittle et al., 1999). The domain that is encoded by exons 1–5 and 8 are present in all VEGF splice variants. The VEGF206 contains all 8 peptide-encoding exons. The VEGF189 and VEGF183 lack some of the peptides that are encoded by exon 6. The VEGF165 lacks the peptides encoded by exons 6, VEGF148 lacks the peptides encoded by exon 6 and part of exon 7, while VEGF145 lacks the peptides encoded by exon 7 and part of exon 6, VEGF121 lacks the peptides encoded by both exons 6 and 7. The VEGF165 is secreted and binds to heparin, rendering it the most frequently studied splice variant.
The VEGF increases vascular permeability; promotes angiogenesis; and enhances survival, proliferation, and migration in various cell types. For example, the differentiation of endothelial cells and cancer cells is regulated by VEGF through an intracrine mechanism (Carmeliet et al., 1996; Ford and D’Amore, 2012; Gordon et al., 2012; Liu et al., 2012; Sitohy et al., 2012). The VEGF mediates vascular inflammation by regulating osteopontin expression (Li et al., 2012c) and contributes to hair growth (Li et al., 2012b). Exogenous VEGF dose-dependently stimulates cell proliferation, which is mediated by vascular endothelial growth factor receptor 2 (VEGFR-2) through phosphorylation of extracellular signal-regulated kinase (ERK) in human outer root sheath cells and human hair follicle dermal papilla cells (Li et al., 2012a; Magnuson et al., 2012). And VEGF expression in secondary hair follicles than it did in primary hair follicles (Zhang et al., 2013). The VEGF accelerates hair growth in mice and humans, but its function has not been determined in goat.
To detect goat VEGF-mediated regrowth of hair, we cloned Inner Mongolia Cashmere goat VEGF164 gene (JX524883.1), which encodes a 190-amino-acid peptide with a signal peptide of 26 amino acids and shows a high homology to VEGF genes in other vertebrates. We then expressed goat VEGF164 (gVEGF164) in E. coli and purified the rgVEGF164 recombinant protein to perform functional studies of gVEGF164. The rgVEGF164 was smeared across a dorsal area of a shaved mouse, and hair regrowth was monitored.
MATERIAL AND METHODS
Molecular cloning of goat VEGF164 gene and transferred into E. coli
Total RNA was isolated using RNAzol (RNAiso Plus, TaKaRa Co. Ltd., Dalian, China) from goat fetal fibroblasts and reverse-transcribed with the AMV 1st Strand cDNA Synthesis kit and an oligo(dT)20 primer per the manufacturer’s instructions (Takara Co. Ltd., China). An input of 1 μg total RNA was used for each reaction.
The gVEGF164 cDNA was amplified by polymerase chain reaction (PCR) with cDNA as template at the appropriate annealing temperature for primers (forward: 5′-ATGAACTTTCTGCTCTCT-3′, reverse: 5′-TCACCGCCTCGGCTTGTC-3′) that contained BamH I (forward) and Hind III (reverse) restriction sites. The amplified cDNA fragment was cloned into pMD19-T (TaKaRa Co. Ltd., China), and the resulting plasmid, pMD19-gVEGF164, was transformed into E. coli DH5α and sequenced on an ABI PRISM 377XL DNA Sequencer (Applied Biosystems, Inc. Foster City, CA, USA). Then, gVEGF164 was subcloned into the pET-his prokaryotic expression vector (Novagen, Inc. Madison, WI, USA) from pMD19-gVEGF164, generating the pET-gVEGF164 expression vector. The pET-gVEGF164 was transformed into E. coli BL21 (DE3) competent cells and confirmed by restriction analysis and sequencing.
Expression of recombinant protein
E. coli BL21 (DE3) cells were transformed with pET-gVEGF164. The expression of 6×his-fused recombinant protein (6×his-gVEGF164) was induced by 0.5 mM isopropyl thio-β-D-galactoside (IPTG) for 5 h at 32°C to an OD600 of 0.6. The expressed recombinant protein was identified by 15% (w/v) sodium dodecyl sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE). Premixed protein marker (TaKaRa Co. Ltd., China) was used as the molecular weight standard. Protein bands were visualized with Coomassie Brilliant Blue R-250 (Sigma-Aldrich, St. Louis, MO, USA), and protein content was measured by Bio-Rad assay (Bio-Rad Laboratories, Hercules, CA, USA). The expressed recombinant protein was named rgVEGF164.
Purification of recombinant goat VEGF164 and SDS-PAGE analysis
The bacterial culture was harvested by centrifugation at 12,000 rpm for 2 min at 4°C, and the pellet was washed twice with 15 mL phosphate buffer saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4). The cells were dissolved in 2 mL cold 1× equilibration/wash buffer (50 mM sodium phosphate, 300 mM NaCl) with 0.75 mg/mL lysozyme, ultrasonicated, and centrifuged at 12,000 rpm at 4°C.
Recombinant rgVEGF164 was purified using the HisTALONGravity Columns Purification Kit (Clontech, Laboratories, Inc., Terra Bella Avenue, Mountain View, CA USA) per the manual, which is a His-tag nickel purification system. Then, the target protein was purified using the Micro Protein PAGE Recovery Kit (SangonBiotech Co., Ltd. Shanghai, China). Finally, the protein was dissolved in 0.1 M phosphate buffer, pH 7.4. The protein lysate supernatants were electrophoresed on 15% (w/v) SDS-polyacrylamide gels, and unstained protein molecular weight marker (MBI Fermentas, Pittsburgh, PA, USA) was used as the molecular weight standard.
Western blot analysis of recombinant goat VEGF164 with monoclonal anti-6×his and anti-vascular endothelial growth factor antibodies
Equal amounts (30 μg) of purified protein were electrophoresed on 15% (w/v) SDS-polyacrylamide gels. Then, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes and incubated with monoclonal anti-His antibody (Cell Signaling Technology, Inc., Danvers, MA, USA) and monoclonal anti-VEGF antibody (Thermo Fisher Sientific Anatomical Pathology, Fremont, CA, USA) overnight at 4°C and horseradish peroxidase -conjugated sheep anti-mouse IgG (GE Healthcare UK Limited, Dorset, UK) at room temperature for 1 h. Signals were detected by chemiluminescence (Amersham).
Induction of hair growth by recombinant goat VEGF164
Eight-week-old wild-type Kunmingbai mice were used to monitor the induction of hair growth by rgVEGF164. 50 μL of rgVEGF164 (1 mg/mL) was applied to a dorsal and lateral area of shaved skin, and control vehicle was applied with isopycnic phosphate-buffered saline to the opposing adjacent area on the same animal). Mice were painted with rgVEGF164 once a day for 1 week, and after that animals were fed for 2 weeks without treatment. The mice were housed under specific pathogen free (SPF) conditions and fed with SPF standards of care.
Statistical analysis
Descriptive statistics were generated for all quantitative data, expressed as mean±SD. For each assay, triplicate parallel observations were examined.
RESULTS
Cloning of goat VEGF164 and construction of pET-gVEGF164 expression vector
PCR of cDNA from goat fetal fibroblasts was performed to amplify a 573-bp fragment that encompassed the full open reading frame of gVEGF164, encoding 190 amino acids. The 26 N-terminal amino acids constitute a signal peptide sequence. The open reading frame (ORF) fragment was amplified and cloned into pMD19-T. The resulting plasmid was designated pMD19-gVEGF164.
The pMD19-gVEGF164 was digested with BamH I and Hind III and subcloned into pET-his to obtain the pET-gVEGF164 expression vector (Figure 1). The pET-gVEGF164 was identified by restriction enzyme analysis, confirmed by sequencing, and transferred into E. coli BL21 (DE3) cells to express the 6×his-gVEGF164 recombinant protein.
Expression, purification, and identification of recombinant 6×his-gVEGF164 fusion protein
Goat VEGF164 was expressed as 6×his-gVEGF164 recombinant protein (rgVEGF164) after induction in E. coli BL21 (DE3) cells that were transformed with pET-gVEGF164. We determined the optimal culture conditions for the rgVEGF164 protein as follows: 0.5 mM IPTG with a 5-h induction at 0.6 of the OD600 value at 32°C. The supernatants of the protein lysates from E. coli BL21 (DE3) cells, the bacterial cells transformed with pET-his and transformed with pET-gVEGF164 were electrophoresed on 15% (w/v) SDS-polyacrylamide gels, and rgVEGF164 was detected by SDS-PAGE (Figure 2A).

Analysis of the expression of rgVEGF164 in E. coli BL21 (DE3). (A) Expression of rgVEGF164 analyzed by 15% SDS-PAGE. Lane 1, lysates of E. coli BL21 (DE3) cells before induction; lane 2, lysates of BL21 cells harboring pET-His before induction; lane 3, lysates of BL21 cells harboring pET-gVEGF164 before induction; lane 4, lysates of BL21 cells after induction; lane 5, BL21 lysates harboring pET-His after induction; lane 6, lysates of BL21 cells harboring pET-gVEGF164 after induction; M, low protein molecular weight marker (D530S, Takara Co. Ltd., Dalian, China). The arrowhead indicates the target protein. (B) Purification and identification of rgVEGF164. Lane 1, purified VEGF164; M, unstained protein molecular weight marker (SM0431, Thermo Fisher Sientific Anatomical Pathology, Fremont, CA, USA). VEGF, vascular endothelial growth factor; rgVEGF164, recombinant goat VEFG164; SDS-PAGE, Sodium dodecyl sulfate-polyacrylamide gelelectrophoresis, gVEGF164, goat VEGF164.
The rgVEGF164 was purified from the total protein lysate of pET-gVEGF164-transformed bacteria and electrophoresed. A single band of approximately 26.0 kDa appeared by 15% (w/v) SDS-PAGE (Figure 2B). After renaturation, the purified protein was identified by western blot using monoclonal anti-His (Figure 3A) and anti-VEGF antibodies (Figure 3B). Purified recombinant 6×his-gVEGF164 was then applied to the dorsal and lateral area of a shaved mouse.

Western blot analysis of rgVEGF164. (A) Western blot analysis of rgVEGF164 with monoclonal anti-His antibody. Lane 1, purified rgVEGF164; M, PM Western Midview Marker (CW0021, Beijing ComWin Biotech Co. Ltd., Beijing, China). (B) Western blot analysis of rgVEGF164 with monoclonal anti-VEGF antibody. Lane 1, purified rgVEGF164; M, PM Western Midview Marker (CW0021, Beijing ComWin Biotech Co. Ltd., China). VEGF, vascular endothelial growth factor; rgVEGF164, recombinant goat VEFG164.
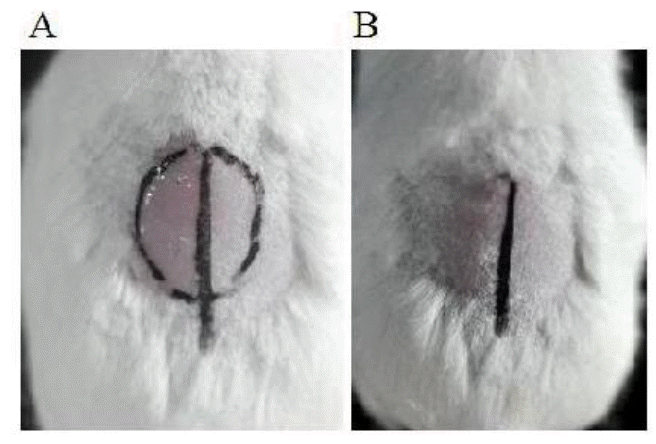
Recombinant goat VEGF164 increases hair growth in mouse
To determine the bioactivity of rgVEGF164 in promoting hair growth, mice were treated with rgVEGF164. Mice were shaved, and rgVEGF164 was applied topically on the shaved area, as described in Methods. The treatment was administered for 7 days in the first week, and after that animals were fed for 2 weeks without treatment. As a result, rgVEGF164-treated, but not control, animals experienced rapid hair regrowth in 3 weeks (Figure 4), indicating that rgVEGF164 promotes hair growth.
DISCUSSION
The hair follicle, a type of epidermal appendage, is composed of dermal papillae cells, epithelial cells of the root sheath, and the hair shaft (Sperling, 1991). The hair follicle life cycle comprises the telogen, anagen, and catagen stages. Hair growth needs perifollicular vascularization, during which VEGF is upregulated in the outer root sheath (Yano et al., 2001) and highly expressed in anagen hair follicles, including the outer and inner root sheaths and dermal papillae epidermal matrix, particularly in the follicular basement membrane zone in normal human skin (Man et al., 2009). The VEGF promotes hair growth, increases the number of hair follicles, and elongates the hair shaft by improving follicle vascularization in mice (Yano et al., 2001; Ozeki and Tabata, 2002).
The effects of VEGF are mediated primarily through its receptors, VEGF receptor-1 (fms-like tyrosine kinase-1), -2 (kinase domain region), and -3 (soluble Flt). The chief receptor of VEGF, VEGFR-2, is believed to mediate most processes, such as cell proliferation, migration, and survival (Ferrara et al., 2003; Holmes et al., 2007; Olsson et al., 2006). Basal cells of the sebaceous glands expressed abundant VEGF and VEGFR-2 (Man et al., 2009), and VEGF active VEGFR-2 in the hair follicle stem cell (Wu et al., 2014), and VEGF165 upregulates VEGFR-2 mRNA and protein and induces phosphorylation of VEGFR-2 in the outer root sheath. Exogenous VEGF stimulates VEGFR-2-mediated proliferation through phosphorylation of ERK in human outer root sheath cells and human hair follicle dermal papilla cells (Li et al., 2012a).
The VEGF-overexpressing transgenic mice experience accelerated hair regrowth compared with wild-type littermates, and have longer and thicker hair after 11 days (Yano, 2001). In contrast, abdominal fur does not regrow properly in rats that have been treated with AEE788, a dual inhibitor of epidermal growth factor receptor (EGFR) and VEGFR, over 4 weeks (Deng et al., 2011). These results demonstrate that VEGF mediates hair growth during development.
In this study, we generated functional rgVEGF164 protein using a prokaryotic expression system, wherein an approximately 26-kDa recombinant protein was detected by monoclonal anti-VEGF. The VEGF has a molecular weight of roughly 45 kDa under nonreducing conditions versus about 23 kDa under reducing conditions (Ferrara and Henzel, 1989). The VEGF is a homodimer that comprises 2 polypeptide chains and has a molecular mass of approximately 45 kDa; the single stranded form has a molecular weight of about 23 kDa under denaturing conditions. We believe that the 6×his-gVEGF164 fusion protein exists in monomeric form—rgVEGF164 can not form a homodimer due to the use of a prokaryotic expression system. Renatured rgVEGF164 was smeared across a dorsal area of a shaved mouse and had a positive effect on hair growth. These results demonstrate that rgVEGF164 increases hair growth in mice.
CONCLUSION
We cloned goat VEGF and expressed it in E. coli BL21 (DE3) cells as a 6×his-tagged fusion protein. rgVEGF was purified and identi ed by western blot using monoclonal anti-VEGF. The rgVEGF164 was applied to the dorsal area of a shaved mouse, accelerating hair regrowth faster than in the control group. Thus, rgVEGF164 effects hair growth in mice.
ACKNOWLEDGMENTS
This work was supported by a grants from the Natural Sciences Foundation of China (No. 31160469, 31360561); Natural Sciences Foundation of Inner Mongolia, China (No. 2011MS0521); a graduate student research project of Inner Mongolia University; and Major Projects for New Varieties of Genetically Modified Organisms (No.2013ZX08008-002).