Evaluation of Houttuynia cordata and Taraxacum officinale on Growth Performance, Nutrient Digestibility, Blood Characteristics, and Fecal Microbial Shedding in Diet for Weaning Pigs
Article information
Abstract
A total of 144 pigs ((Landrace×Yorkshire)×Duroc] with an average initial BW of 8.45±0.57 kg were used in a 5-wk growth trial. Pigs were randomly allocated to 4 treatments with 9 replications per pen in a randomized complex block design. Dietary treatments included: i) CON (basal diet), ii) ANT (CON+tylosin 1 g/kg), iii) H1 (CON+H. cordata 1 g/kg) and iv) T1 (CON+T. officinale 1 g/kg). In this study, pigs fed the ANT and T1 treatment had a higher (p<0.05) average daily gain (ADG) and gain:feed (G:F) ratio than those fed CON and H1 treatment. Dietary ANT and T1 treatment led to a higher energy digestibility than the CON group. No difference (p>0.05) was observed on the growth performance and apparent total tract digestibility with H1 supplementation compared with the CON treatment. The inclusion of ANT treatment led to a higher (p<0.05) lymphocyte concentration compared with the CON treatment. Dietary supplementation of herbs did not affect (p>0.05) the blood characteristics (white blood cell (WBC), red blood cell (RBC), IgG, lymphocyte). No difference was observed on (p<0.05) fecal microbial shedding (E. coli and lactobacillus) between ANT and CON groups. Treatments H1 and T1 reduced the fecal E. coli concentration compared with the CON treatment, whereas the fecal lactobacillus concentration was not affected by the herb supplementation (p>0.05). In conclusion, the inclusion of T. officinale (1 g/kg) increased growth performance, feed efficiency, energy digestibility similarly to the antibiotic treatment. Dietary supplementation of T. officinale and H. cordata (1 g/kg) reduced the fecal E. coli concentration in weaning pigs.
INTRODUCTION
The ban of antibiotics utilization as feed additives in livestock resulted in a great interest on the antibiotics alternative in livestock industry. Among this, medicinal herbs was considered to be the a good one because of its stimulate effect on appetite and secretion of digestive enzymes (Wenk, 2003; Cho et al., 2006; Huang et al., 2010). Thus, a lot of medicinal herbs have been used as feed additives in pig production industry to stimulate the animal growth performance (Huang et al., 2010; Yan et al., 2010, Ao et al., 2011; Yan et al., 2011a, b; Yan et al., 2012 a; Yan and Kim, 2012).
Houttuynia cordata (H. cordata) have been used in many traditional medicines because of their antimicrobial, antiviral and anti-inflammatory properties (Chang et al., 2001; Chiang et al., 2003). Kim et al. (2007) had previously suggested that H. cordata may be beneficial for the treatment of mast cell-mediated inflammation. Yan et al. (2011b) also suggested that the inclusion of H. cordata could increase the growth performance and nutrient digestibility in finishing pigs. Therefore, we hypothesized the inclusion of H. cordata extract powder could also benefit the weaning pig by improving their health status.
Taraxacum officinale (T. officinale) has also been used as medical herb for several human or animals for a long time because of its anti-inflammatory, anti-oxidative, anti-allergic activity (Ho et al., 1998; Hagymasi et al., 2000). Our previous study (Yan et al., 2011b) had suggested that the inclusion of T. officinale could increase the growth performance and gut health in finishing pigs. Trojanova et al. (2004) also suggested that T. officinale could be used as a prebiotic in vitro.
The objective of our study was to evaluate the effect of H. cordata and T. officinale extract powder supplementation on growth performance, apparent total tract digestibility (ATTD), blood characteristics and fecal microbial shedding in weaning pigs.
MATERIALS AND METHODS
The experimental protocols were approved by the Animal Care and Use Committee of Dankook University (Cheonan, Choognam, Korea).
Preparation of herb extracts mixture
The dried plant leaves of H. cordata and T. officinale were chopped and pulverized to pass 100 mesh (2 mm). An extract of the herb was prepared as described by Jang et al. (2008). Briefly, 100 kg of each powdered medicinal herb was extracted overnight with 200 L of 75% methanol by using a large-scale extractor at room temperature. The 75% methanol solution was filtered 2 to 3 times with cheesecloth, and the filtrate was concentrated by a rotary evaporator under vacuum, freeze-dried and crushed in the form of powder form.
Experimental design, animals, and facilities
A total of 144 pigs ((Landrace×Yorkshire)×Duroc) with an average initial BW of 8.45±0.57 kg were used in a 5-wk growth trial. Pigs were randomly allocated to 4 treatments with 9 replications (Pens) each consisting of 4 pigs (two barrows and two gilts) in a randomized complex block design according to its BW and sex. Dietary treatments included: i) CON (basal diet), ii) ANT (CON+tylosin 1g/kg), iii) H1 (CON+H. cordata 1 g/kg) and iv) T1 (CON+T. officinale 1 g/kg). A 3-period feeding program was employed in the current experiment (Table 1), which consisted of phase 1 (0 to 1 wk), phase 2 (2 to 3 wks), phase 3 (4 to 5 wks). All diets used in the present study were formulated to meet or exceed the nutrient recommendations of the NRC (1998). The additive was supplemented in the diet by replacing the same amount of corn. The pigs were housed in an environmentally controlled nursery room. The stainless steel pens were 0.6×2.0 m with a slatted plastic floor and a cage height of 0.5 m. Each pen was provided with a stainless steel feeder and a nipple drinker that allowed for ad libitum access to feed and water throughout the experiment. Ventilation was provided by a mechanical system, and lighting was automatically regulated to provide 12 h of artificial light per day. The ambient temperature within the room was approximately 30°C and decreased by 1°C each wk of the experiment.
Sampling and measurements
The individual pig BW and feed consumption (weekly) of each pen was monitored to calculate average daily gain (ADG), average daily feed intake (ADFI), and gain:feed ratio (G:F). Chromium oxide (Cr2O3, 2 g/kg) was added to the diets as an indigestible marker on d 29 to measure ATTD. Fresh fecal grab samples were obtained from at least two pigs (1 gilt and 1 barrow) in each pen on d 35 to determine the apparent digestibility of dry matter (DM), nitrogen (N), and energy. All feed and feces samples were stored immediately at −20°C until analysis. Fecal samples were dried at 70°C for 72 h and finely ground to pass through a 1-mm screen. The procedures utilized for the determination of DM and N digestibility were conducted in accordance with the methods established by the AOAC (2000). Chromium levels were determined via UV absorption spectrophotometry (UV-1201, Shimadzu, Kyoto, Japan) and the apparent total tract digestibility (ATTD) of DM and N were calculated using indirect methods described by Williams et al. (1962). The gross energy was determined by measuring the heat of combustion in the samples using a Parr 6100 oxygen bomb calorimeter (Parr instrument Co., Moline, IL, USA).
At the beginning of the experiment, two pigs (one gilt and one barrow) were randomly selected from each pen and bled via jugular venipuncture using a sterile needle into either a 5-ml or a K3EDTA tube for subsequent analysis (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA). The same pigs were then bled again at the end of the experiment, after which the serum was separated by centrifugation for 30 min at 2,000×g and the aliquot was stored at −4°C until it was analyzed for IgG using an automatic biochemistry blood analyzer (HITACHI 747, Hitachi, Tokyo, Japan). The red blood cells (RBC), white blood cells (WBC), and lymphocyte counts of the whole blood samples were determined using an automatic blood analyzer (ADVIA 120, Bayer, Tarrytown, NY, USA).
At d 35, fecal samples were collected via massaging the rectum from 2 pigs randomly selected from each pen (1 gilt and 1 barrow) and pooled and placed on ice for transportation to the laboratory, where analysis was immediately carried out. The composite fecal sample (1 g) from each pen was diluted with 9 ml of 1% peptone broth (Becton, Dickinson and Co.) and homogenized. Viable counts of bacteria in the fecal samples were then determined by plating serial 10-fold dilutions (in 1% peptone solution) onto MacConkey agar plates (Difco Laboratories, Detroit, MI, USA) and lactobacilli medium III agar plates (Medium 638, DSMZ, Braunschweig, Germany) to isolate E. coli and Lactobacillus, respectively. The lactobacilli medium III agar plates were then incubated for 48 h at 39°C under anaerobic conditions. The MacConkey agar plates were incubated for 24 h at 37°C. Escherichia coli and Lactobacillus colonies were counted immediately after removal from the incubator.
Statistical analyses
Data were analyzed by ANOVA using the General Linear Models (GLM) procedure of SAS (SAS Institute, 1996), with the pen being defined as the experimental unit. Differences among treatments were separated by Duncan’s multiple range test. The results were expressed as the least squares means and SE. Probability values less than 0.05 were considered significant.
RESULTS
Growth performance and ATTD
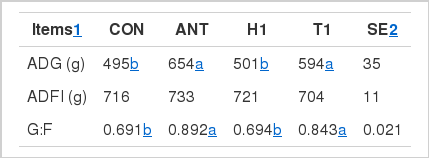
Pig fed the ANT and T1 treatment led to a higher (p<0.05) ADG and G:F ratio than those fed CON and H1 treatment. The inclusion of H1 did not affect (p>0.05) the ADG compared with the CON treatment. Dietary ANT and T1 treatment led to a higher energy digestibility than the CON group. No difference (p>0.05) was observed on the apparent total tract digestibility with H1 supplementation compared with the CON treatment.
Blood characteristics
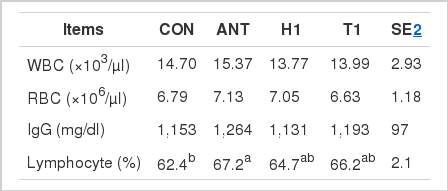
The inclusion of ANT treatment led to a higher (p<0.05) lymphocyte concentration compared with the CON treatment. Dietary supplementation of herb did not affect (p>0.05) the blood characteristics (WBC, RBC, IgG lymphocyte) throughout the experiment.
Fecal microbial shedding
Pigs fed antibiotic supplemental diets did not affect (p<0.05) the fecal microbial shedding (E. coli and lactobacillus) at the end of this study (Table 5). The inclusion of H1 and T1 reduced the fecal E. coli concentration compared with the CON treatment, whereas the fecal lactobacillus concentration was not affected with the herb supplementation (p>0.05).
DISCUSSION
Growth performance and nutrient digestibility
It is well accepted that antibiotic supplementation could greatly improve the growth performance of swine because of its antimicrobial effect and health promoting effect (Yan et al., 2011a). Wang et al. (2011) had previously suggested that the inclusion of antibiotic could improve the growth performance of growing pig. Our previous study (Yan et al., 2011c) also suggested that pig fed antibiotic supplemental diet could improve the growth performance and digestibility compared with those fed normal diet. Similarly in this study, the inclusion of antibiotics led to a higher ADG and G:F, which again confirmed the antibiotic effect on the weaning pigs.
In terms of the herbal feed additives, the inclusion of T1 treatment led to higher ADG than the CON treatment, which is in agreement with our previous study (Yan et al., 2011b), who suggested that dietary T1 treatment increased the ADG of finishing pigs. However, it should be noted that the ADFI was also increased with T1 supplementation in that study, which was not the case in this study. But interestingly, dietary T1 treatment increased the G:F ratio compared with CON treatment, indicating the reason for the improved ADG was not its beneficial effect on feed intake, but its improved nutrient utilization. Indeed, supplementation of T. officinale increased the energy utilization compared with the CON group, which confirm its effect on the energy utilization. Similarly, our previous result (Yan et al., 2010) also suggested that dietary plant essential oil increased the feed efficiency and nutrient digestibility in grower and finisher pigs. But in contrast, pig fed the H1 treatment did not affect the growth performance throughout the experiment, which is inconsistent with our previous study in finishing pig (Yan et al., 2011a), who suggested that the inclusion of H. cordata increased the ADG and ADFI compared with the CON group. Since the pigs age used in those two studies were different; therefore, we hypothesized that the reason for the difference is likely to be the different animal and different age used in each study. However, further study is still necessary to investigate its exactly mechanism before applying this herb in swine industry.
Blood characteristics
In the present study, dietary antibiotic led to a higher lymphocyte concentration than the control treatment, which is in agreement with our previous study (Yan et al., 2011b), who suggested that the inclusion of antibiotic increased the lymphocyte concentration. It is well suggested that gastrointestinal tract together with its associated lymphoid system are the largest immunologically competent organ in the body (Michael, 1988). Therefore, we hypothesized the beneficial effect of antibiotic on the lymphocyte concentration may be attributed to the improved gut health caused by the antibiotic supplementation.
However, supplementation of H1 and T1 did not significantly affect the lymphocyte concentration, although there were a numerically increase. In agreement with this study, our previous study (Yan et al., 2011b) also suggested that the supplementation of H. cordata or T. officinale did not affect the lymphocytes concentration in growing pigs. But in contrast, Kong et al. (2007) had suggested that the inclusion of herbal ultra-fine powder enhanced the production of cytokines and lymphocyte proliferating activity in piglets. Yan et al. (2011a) also suggested that the inclusion of herb extract mixture increased the lymphocyte concentration in finishing pig. The reason for the difference is likely to be the different herb used in different studies. Therefore, this study together with our previous study (Yan et al., 2011b) confirmed that the inclusion of H. cordata or T. officinale will not affect the lymphocyte concentration in pigs.
Fecal microbial shedding
Previously, it is well suggested that herbs and spices could significantly affect the pathogens in vitro because of its antimicrobial actions (Si et al., 2006). Windisch et al. (2008) had suggested that herbs and spices could influence pathogenic microorganisms’ growth in the gastrointestinal ecosystem, and subsequently increase the resistance of the animal exposed to different stress situations. Other authors (Insoft et al., 2005; Michael and Marteau, 2007) also suggested that the maturation and optimal development of immune system are highly related to the development and composition of the indigenous microflora and vice versa. Therefore, the fecal microbial shedding was investigated in this study. Our results indicated that supplementation of H1 and T1 significantly reduced the E. coli concentration compared with the control treatment, which is in agreement with Jugl-Chizzola et al. (2005), who suggested thyme supplementation could decrease the fecal E. coli concentration in piglets. However, some studies have failed to find effect of phytogenic compound on the fecal shedding of specific pathogens (Namkung et al., 2004; Hagmüller et al., 2006), and concluded that the reason for the discrepancies may be due to the differences in the quality of herbal materials, selection of particular herbs and forms of their administration (Windisch et al., 2008).
CONCLUSION
In conclusion, the inclusion of T. officinale supplementation (1 g/kg) increased growth performance, feed efficiency, energy digestibility similarly to the antibiotic treatment. Dietary supplementation of T. officinale and H. cordata (1 g/kg) reduced the fecal E. coli concentration in weaning pig due to its antimicrobial effect.
ACKNOWLEDGEMENT
This work was supported by the grant from the institute of Bio-Science and Technology (IBST) AT Dankook University in 2012.