Indirect Estimation of CH4 from Livestock Feeds through TOCs Evaluation
Article information
Abstract
Thirty-five available feeds were fermented in vitro in order to investigate their soluble total organic carbon (TOCs) and methane (CH4) production rate. A fermentation reactor was designed to capture the CH4 gas emitted and to collect liquor from the reactor during in vitro fermentation. The results showed that CH4 production rate greatly varied among feeds with different ingredients. The lowest CH4-producing feeds were corn gluten feed, brewer’s grain, and orchard grass among the energy, protein, and forage feed groups, respectively. Significant differences (p<0.05) were found in digestibility, soluble total organic carbon (TOCs), and CH4 emissions among feeds, during 48 h of in vitro fermentation. Digestibility and TOCs was not found to be related due to different fermentation pattern of each but TOCs production was directly proportional to CH4 production (y = 0.0076x, r2 = 0.83). From this in vitro study, TOCs production could be used as an indirect index for estimation of CH4 emission from feed ingredients.
INTRODUCTION
Ruminants depend on plant source feed that is digested anaerobically in their rumen through microbial enzymes. Volatile fatty acid (VFA) and other organic acids are the primary energy sources in rumen fermentation. Microbial fermentation in the rumen also produces waste products such as CH4 and carbon dioxide (CO2). CH4 production in the rumen is an energetically wasteful process that reduces the efficiency of feed utilization. Approximately 2 to 12% of the dietary gross intake energy of feed is lost to the atmosphere as CH4 (Moss et al., 2000; Kumar et al., 2009; Yurtseven et al., 2009). Knowledge regarding ruminant feed digestion kinetics is necessary for a better understanding of the pattern of degradation and CH4 emission. In vitro digestion technique is a rather simple method of feed evaluation, and a large number of feeds can be simultaneously incubated and analyzed. Therefore, it is the most appropriate and widely utilized technique to measure gas emission from livestock feed ingredients. When feedstuff is incubated with buffered rumen fluid in vitro, carbohydrates are fermented by microbial cells to short-chain fatty acids and gases. Gas production is essentially the result of fermentation of carbohydrate to acetate, propionate, and butyrate (Blummel and Orskov, 1993; Getachew et al., 1998). The amount of gas produced due to protein fermentation is smaller than that produced due to carbohydrate fermentation. Carbohydrate is the chiefsource of acetate and butyrate in rumen fermentation. The synthesis of acetate and butyrate in the rumen increases hydrogen production (Widiawati and Thalib, 2007), and the methanogenic bacteria in the rumen enhance CH4 production by utilizing hydrogen and CO2.
Increased production of acetate and CO2 leads to increased CH4 production, which represents a net loss of feed energy as well as inefficient feed utilization. In contrast, increased propionate production reduces CH4 emission in the rumen. CO2 production from carbohydrate fermentation contributes to approximately 40% of total gas production in the rumen (Mcdonald et al., 1995); therefore, feeds containing large amounts of carbohydrate should produce more gas than feeds containing less amounts of carbohydrate. Amount of feed intake, feed composition, and organic matter degradability are also related to CH4 and CO2 production (Monteny et al., 2006): higher feed intake reduces digestibility and increases CH4 and CO2 production.
Since, volatile fatty acids and other organic carbon sources represents the soluble total organic carbons, there could be a possibility of correlation between CH4 production rates from feed ingredients and total organic carbons. Therefore, the rates of CH4 emission and other fermentation parameters of 35 available feed ingredients were assessed by in vitro fermentation in the present study and the interrelationship between CH4 emission and total organic carbons (TOCs) levels was studied to determine the correlation for the indirect estimation of CH4 from livestock feeds.
MATERIALS AND METHODS
Apparatus for in vitro fermentation
Two types of fermentation reactors were designed to collect liquid samples, to capture CH4 gas emitted during the in vitro test, and to analyze TOCs production during fermentation. One reactor (500 ml) was connected to a tedlar bag to capture gases, and the other reactor was equipped with a 50-ml syringe and tube to collect liquor samples during fermentation A set of 210 reactors, containing one bag in one reactor, were placed in 6 shaking incubators and three replicates of each were tested at the same time. Fermentation was performed in a shaking incubator (VS-8480 SR) to avoid settling of feed particles and to ensure proper physiological function of the microorganisms. In vitro fermentation of the feeds was carried out according to the principles of Tilley and Terry (1963).
Preparation for in vitro fermentation (feed sample, inoculums, and incubation)
In vitro fermentation of the 35 available feed ingredients (16 energy-rich and 11 protein-rich feeds and 8 roughages) was performed to assess CH4 production rate. The detailed composition of the experimental feed ingredients is shown in Table 1. Clean, dry, nylon bags (mesh size: 30–50 μm; dimension: 5×10 cm) were rinsed in acetone for 3 to 4 min and completely air-dried before sampling. After measuring the weight of the nylon bag, 1.6 g of basal feed (as the correction factor and for optimizing the best ecosystem conditions for microbial growth and rapid colonization) and 2.4 g of dried and ground experimental feed (1.0–2.0 mm) were put into each bag. The basal feed comprised 0.8 g ground rice straw and 0.8 g formula feed. The nylon bags were sealed after adding 4 beads to each bag to ensure complete immersion in the Menke buffer solution. Prepared nylon bags (2 each) with feed samples were placed in the marked fermentation reactor for fermentation. Buffer solution was prepared according to the method described by Menke and Steingass (1988), and the pH was adjusted to 6.8. The buffer solution (320 ml) was added to each fermentation reactor and warmed to 39°C for 20 to 30 min; thereafter, rumen inoculum (80 ml) was added to each reactor. All the 35 ingredients were tested in the same batch, with same media and inocula and in triplicates. Rumen fluid and contents were collected from fistulated non lactating Korean cattle (maintained at National Institute of Animal Science on a standard diet concentrate:roughage = 40:60) approximately 30 min after feeding and placed in a O2-free CO2 flushed, pre-warmed insulated container. Anaerobic conditions were maintained by injecting CO2 gas, and the liquor was homogenized by blending at high speed for 30 s. Homogenization was required to dislodge microbes attached to the fibrous mat and ensure an adequate microbial population for in vitro analysis. Homogenized digesta (liquor) was filtered through four-layered cheesecloth and continually purged with CO2. After addition of the liquor to the reactors with buffer solution and nylon bag with feed samples, the tedlar bags were tightly installed in the reactors. The reactors were then incubated at 39°C and stirred at 170 rpm for 48 h by placing the reactor on shaker incubator to avoid settling of feed particles and ensure proper physiological function of the microorganisms.
Sampling and analysis
Liquor samples were collected using the installed syringe at 0, 12, 24, and 48 h to analyze the TOCs levels. TOCs production was analyzed by a Total Organic Carbon Analyzer (Shimadzu, TOC-5000A). Methane was analyzed from the total volume of the gases collected in the tedlar bag by injecting 60 ml of gas into a GC (Varian, 450-GC) equipped with a thermal conductivity detector. Digestibility of feeds was measured by drying the nylon bags washed with cold tap water at 60°C for 4 d and by weighing the residual feeds. All feed analyses (ash, EE, CP, CF, ADF and NDF) were done according to standard methods (AOAC, 2005).
The methane values were converted in g/kg feed as:
Where, 250 is used as a factor to convert the 4 g feed into Kg.
Where, conversion factor is 1,000/digested feed weight.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 12.0, 2003), a computer statistical package program with one-way analysis of variance (ANOVA). Differences among treatment mean values were determined by the Tukey Multiple Range Test (MRT) according to the principles of Steel and Torrie (1980).
RESULTS AND DISCUSSION
CH4 production rate of feeds
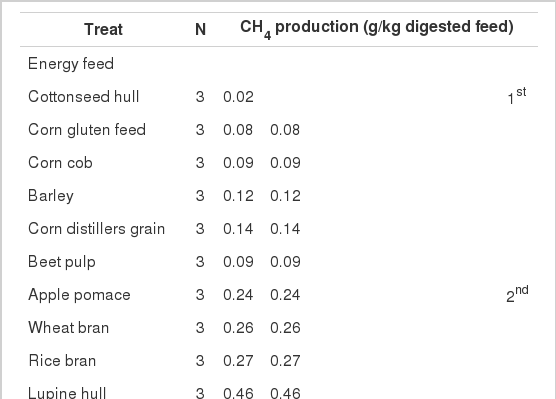
Experimental feeds were categorized according to the Tukey MRT value of CH4 emitted during 48 h of in vitro fermentation (Table 2). In the case of energy feed, there were 6 distinct categories of feed ingredients regarding CH4 emission. The CH4 emission rates were higher in the case of wheat (8.29 g/kg digested feed) and thus placed in the sixth category. The fifth-category feed included tapioca (4.59 g/kg digested feed) and fourth and third categories include soybean hull, USA corn and Latin American corn emitting CH4 in range of 2.28–3.18 g/kg digested feed. Second category feed (rye, lupine hull, apple pomace, corn distiller’s grain, corn cob, barley, beet pulp, rice bran, wheat bran, and corn gluten feed) emitted lower CH4 levels during 48 h of in vitro fermentation (CH4 emission rate, 0.08–0.54 g/kg digested feed). Cottonseed hull emitted the lowest amount of CH4 gas (0.02 g/kg digested feed) among all energy feeds and was ranked first. Differences in CH4 emissions were significant among the categories (p<0.05) of the energy feeds.
In the case of protein feeds, soybean oilcake and lupine produced the highest CH4 levels (7.09–7.33 g/kg digested feed) followed by soybean meal and palm cake. Methane emissions from corn gluten meal, rape seed meal, coconut meal, cottonseed meal and corn cake were in range of 0.18 to 0.76 g/kg digested feed, and were lower. Although there was no statistical difference between the second category and lower CH4-emission groups, significant differences were found between the highest group and the lowest and intermediate groups (p<0.05). Brewer’s grain produced the lowest CH4 levels (0.01 g/kg digested feed) and was ranked first among the protein feeds.
Amongst the forages, crain grass produced the highest level of CH4 (5.04 g/kg digested feed). Lowest CH4 emission rate was observed in the case of timothy, tallfescue, oat, perennial grass, rye grass, orchard grass, and alfalfa (0.06–0.67 g/kg digested feed). Differences were significant between the forage feeds with the highest and lowest CH4 levels (p<0.05). The results of the present study mostly supported by the findings of Rossi et al. (2001) regarding CH4 emission and feed quality, wherein corn silage was found to produce the highest CH4 levels, and rye grass produced the lowest CH4 levels among all the forage feeds. Furthermore, their data indicated that for energy feeds, beet pulp and manioca produced the highest and rice bran produced the lowest CH4 levels. Whole soybean and soybean meal produced the highest and cotton meal produced the lowest CH4 levels among all protein feed ingredients during in vitro fermentation.
Relationship between CH4 emission and TOCs
Since different ingredients in the categories of energy, protein and forage feed, have different fermentation pattern (Table 1) hence no relationship between digestibility and TOCs production could be established. Fermentation pattern of proteins/protein rich diets results in both amino acids and short chain peptides which can end up either in microbial biomass or in fermentation end products such as VFA, CO2, or ammonia, which is quite different with that of carbohydrate based diet (Cone and Van Geldar, 1999). Thus results obtained are in accordance with the previous study but fails to establish any relationship between TOCs and digestibility.
During in vitro fermentation, metabolism of TOCs occurs in the microbial body to produce new cells. Short-chain fatty acids, gases, and new microbial cells are produced from carbohydrate sources during in vitro fermentation (Getachew et al., 1998 and 2006). TOCs are the backbone of simple sugars, VFAs, and amino acids. Low TOCs production from highly digestible feeds might be due to the frequent utilization of TOCs for microbial assimilation and synthesis of new cells whereas, diets having high TOCs and better digestibility leads to more CH4 production. Feed having high TOCs may get digested with the help of the complete rumen consortia as rumen fungi takes more time to colonize and degrade the feed ingredients with their array of hydrolytic enzymes and thus contributes to slow and efficient digestion. This leads to slow release of hydrogen and more interspecies hydrogen transfer and thus CH4 production (Kamra, 2005).
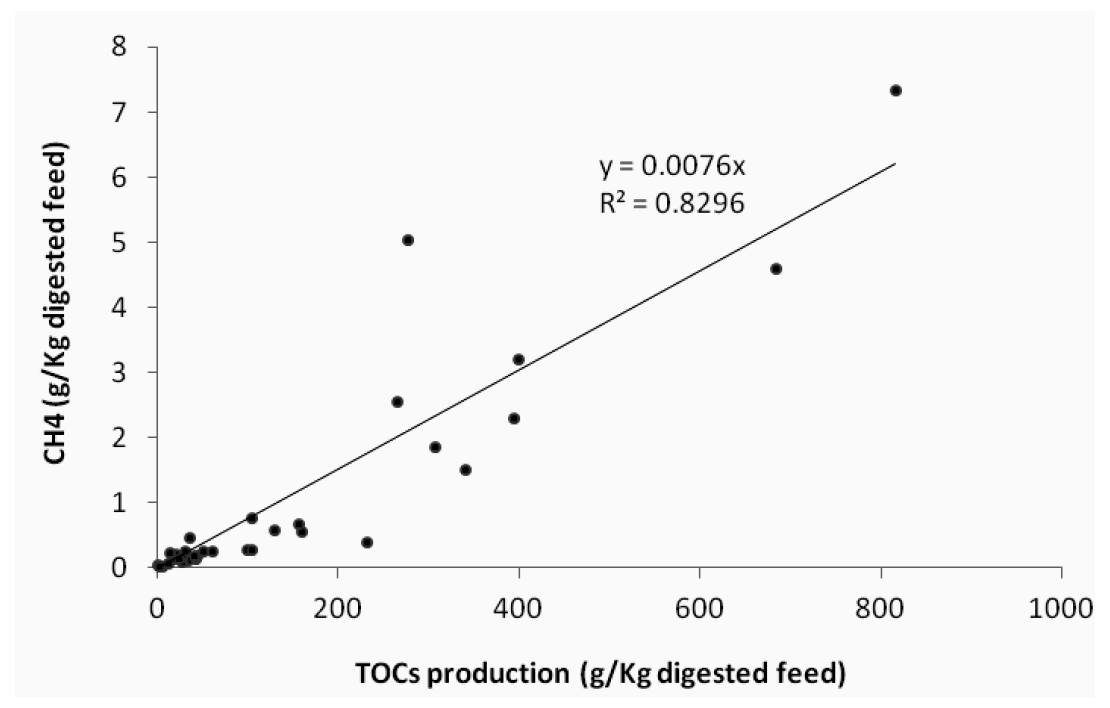
TOCs levels during in vitro fermentation were directly proportional to CH4 production (Table 2 and Figure 1); thus, the amount of CH4 could be estimated from the amount of TOCs produced during in vitro fermentation. Furthermore, TOCs levels could be used as an indirect index to measure CH4 in the ruminal fermentation process (y = 0.0076x, r2 = 0.83).
CONCLUSION
CH4 emission of feed ingredients during in vitro fermentation and interrelationships among digestibility, TOCs, and CH4 emission were examined in this study. The following conclusions were made on the basis of our results:
CH4 emission during in vitro fermentation varied among feed ingredients. Tapioca, soybean hull, wheat, soybean oilcake, lupine, and crain grass resulted in the highest CH4 production levels among the 35 tested feed ingredients. Cottonseed hull, corn gluten feed, brewer’s grain, whole cottonseed, and orchard grass resulted in the lowest CH4 production levels.
In vitro fermentation of the feeds showed that there was no relation between digestibility, TOCs levels and CH4 production. TOCs levels could be used as an indirect index to measure CH4 levels in ruminal fermentation because TOCs levels during in vitro fermentation were directly proportional to CH4 emission.
ACKNOWLEDGEMENTS
We are grateful to the funding by Rural Development Administration, Korea. Also, this work was supported in part by a grant from the Institute of Animal Resources at Kangwon National University.