Blood haematology, muscle pH and serum cortisol changes in pigs with different levels of drip loss
Article information
Abstract
Objective
An experiment was conducted to study the blood haematology, muscle pH, and serum cortisol changes in pigs with different levels of drip loss.
Methods
Two groups (low and high) of 20 animals were selected from 100 pigs based on drip loss. All [Duroc× (Large White×Landrace)] pigs were slaughtered according to standard slaughtering procedures. At exsanguinations, blood samples were taken for the haematological parameters and serum cortisol analysis. The muscle samples were taken from longissimus dorsi muscle to evaluate the muscle pH and drip loss.
Results
Haematological parameters of low drip loss group showed higher content of white blood cells and monocytes than high drip loss group (p<0.05). The low drip loss group had higher muscle pH at 45 min (p<0.05) and 24 h (p<0.001) post-mortem than the high drip loss group. However, there was no significant difference in serum cortisol levels (p>0.05).
Conclusion
Drip loss is mainly affected by the muscle pH decline after slaughter and also might be affected by white blood cells and monocytes.
INTRODUCTION
Meat quality is an indicator of acceptability or preference for the consumer. The important quality traits for fresh meat consist of color, water-holding capacity, texture and fat distribution [1]. Especially, water-holding capacity determined by drip loss is a main quality attribute of fresh meat. Drip loss has financial implications due to the loss of weight, reduced acceptance and rejection by consumers [2]. The large variations are affected by many factors in the whole meat production chain including physiological factors, rearing conditions and processing factors [2]. Particularly, the rate of pH decline is the key factor that correlates to drip loss [3,4]. pH is mainly influenced by pre-slaughter stress [5]. Stress before slaughter can cause a faster muscle glycogen breakdown and thus lower pH values in the muscle when the temperature of the carcass is still high. The combination of low pH and high temperature causes the denaturation of muscle proteins leading to reduction in the meat water holding capacity [6].
Haematology blood parameters are good indicators of the physiological and health status in animals [7,8]. It has been reported that haematological parameters could be employed to highlight the stress condition during transport [9,10]. Decrease in stress has been documented as one of the factors influencing on heterophils, lymphocytes and total white blood count levels [11]. Furthermore, cortisol is the main hormone of the hypothalamic-pituitary-adrenocortical (HPA) axis responding to stress [12]. Increase of serum cortisol was observed in pigs under stress conditions including high temperature and transportation [13]. Increased cortisol levels lead to increase post-mortem metabolism that could be reflected on meat quality [14]. Higher serum cortisol was related poor pork quality with a higher muscle temperature [15], faster muscle pH decline, higher drip loss and lightness in pork [16]. Therefore, the objective of this study was to investigate the blood haematology, pH muscle and serum cortisol changes in pigs with different levels of drip loss to obtain evidence that could be used for prediction water-holding capacity in meat.
MATERIALS AND METHODS
Animals and muscle sampling
A total of 100 three crossbred pigs [Duroc×(Large White× Landrace)] were obtained from a commercial slaughterhouse in Thailand. All pigs were slaughtered according to standard slaughtering procedures. The average of slaughter weight was 112.13± 4.81 kg. After electrical stunning, carcasses were scalded, cleaned, eviscerated and split. Two groups (low and high) of 20 animals were selected from 100 pigs based on drip loss. The muscle samples were immediately taken from the longissimus dorsi muscles at the 2nd to 6th of lumbar vertebrae for further analysis.
Blood haematological and cortisol analysis
Blood samples were collected from each pig (n = 20) within 30 s during exsanguination after electrical stunning and sticking. The blood samples were collected using two types of tubes: the first tube was treated with ethylenediamine tetra-acetic acid (EDTA) to prevent blood coagulation, and the second tube was collected without EDTA. The first tube was used for haematological analysis including red blood cells (RBC), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets, and white blood cells (WBC). These parameters were analyzed with hematological analyzer (Cell Dyn 3700, Wiesbaden, Hesse, Germany). The non-anticoagulated blood (second tube) was centrifuged at 3,000 rpm for 10 min to separate the serum. The serum samples were collected into microtubes and stored at −20°C until the determination of cortisol concentration by chemiluminescent microparticle immunoassay using IMMULITE 1000 automated immunoassay system (Siemens Healthcare Diagnostics Inc., Flanders, NJ, USA).
Muscle pH and drip loss analysis
The longissimus dorsi muscle pH was measured at 45 min and 24 h post-mortem using a pH meter with a spear-type electrode (pH Spear, Eutech Instruments, Singapore City, Singapore). Drip loss was scored base on a bag method with a size-standardized sample from longissimus dorsi muscle collected at 24 h post-mortem that was weighed, suspended in a plastic bag, held at 4°C for 48 h, and thereafter re-weighed. Drip loss was expressed as a percentage [17].
Statistical analysis
Haematological parameters and cortisol levels were described by descriptive statistics (mean, standard deviation, minimum, and maximum value). The effect of low and high drip loss groups on blood haematological parameters, muscle pH and cortisol levels was analyzed by t-tests of SAS (SAS Inst. Inc., Cary, NC, USA). Values of p<0.05 were considered to indicate statistically significant differences. The results are presented as least squares means with the standard errors.
RESULTS
Based on the data obtained, two drip loss groups were identified: low (<1.70%; n = 10) and high (>4.00%; n = 10) drip loss. Mean of the low and high drip loss groups was 1.19%±0.20% and 6.04% ±0.33%, respectively.
Blood haematological parameters
The mean values, standard deviations and overall ranges for blood haematological parameters of the pig population are shown in Table 1. The differences in values of haematological parameters between drip loss groups are presented in Table 2. The significant differences were observed in WBC and monocyte count (p<0.05). The low drip loss group had higher WBC (22.67±2.58 vs 14.93± 1.15×103/mm3, respectively) and monocyte count (3.30%±0.30% vs 2.40%±0.27%, respectively). However, there were no significant differences between drip loss groups in RBC, hemoglobin, haematocrit, MCV, MCH, MCHC, platelets, neutrophils, eosinophils, lymphocytes and neutrophil:lymphocyte ratio.
Muscle pH
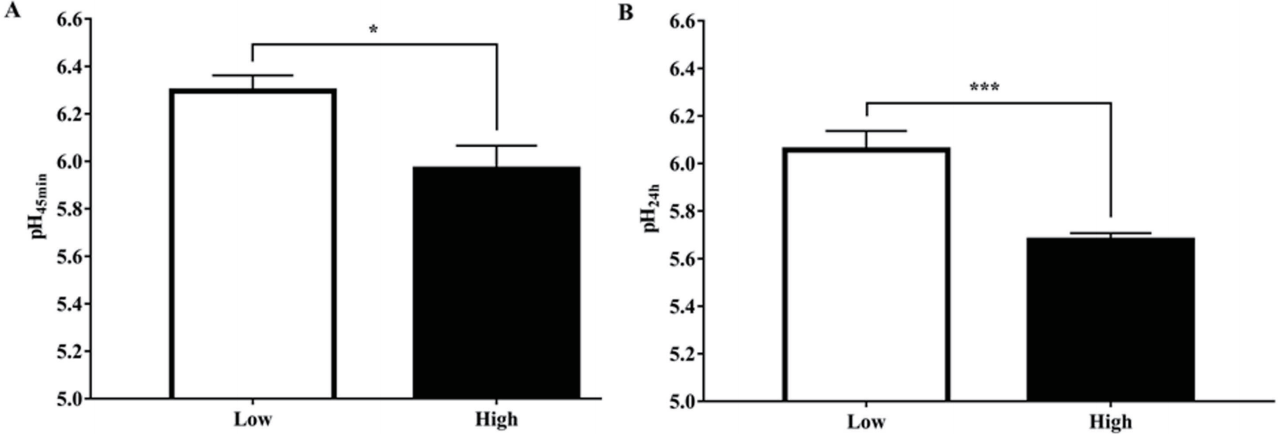
Muscle pH values in the low and high drip loss groups are presented in Figure 1. There were significant differences both in muscle pH at 45 min and 24 h post-mortem. The low drip loss group had a higher muscle pH at 45 min post-mortem than the high drip loss group (p<0.05). At 24 h post-mortem, the low drip loss group was at a higher muscle pH than the high drip loss group (p<0.001).
Serum cortisol levels
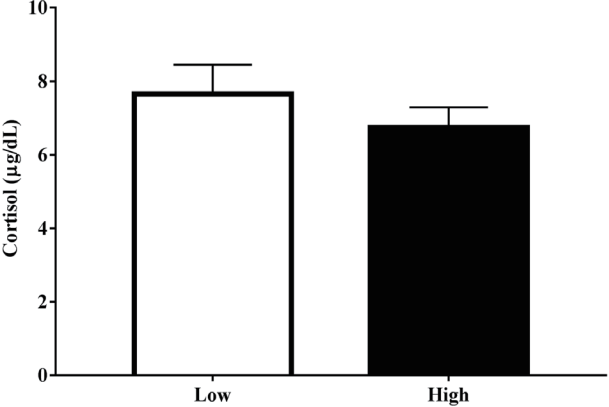
The result for serum cortisol levels for each group is shown in Figure 2. The low and high drip loss group had 7.66±0.80 and 6.74±0.55 μg/dL of serum cortisol, respectively. There was no significant difference in serum cortisol levels between drip loss groups.
DISCUSSION
Blood haematological parameters
Blood haematological parameters are important in assessing the response of animals to various physiological situations [8]. Merck manual reported the normal range of values for pigs as follows: RBC, 5 to 8×106/mm3; hemoglobin, 10 to 16 g/dL; hematocrit, 36% to 43%; MCV, 50 to 68 fL; MCH, 17 to 21 pg; MCHC, 30 to 34 g/dL; platelets, 200 to 500×103/mm3; WBC, 11 to 22×103/mm3; neutrophils, 28% to 47%; eosinophils, 0.5% to 11%; lymphocytes, 39% to 62%; and monocytes, 2% to 10%. The average haematological parameters in this study were represented in the normal range except for neutrophils and lymphocytes. There were higher mean values of neutrophils and lower lymphocytes values in comparison to the normal range. This may have been influenced by stress prior to slaughter. An increase in neutrophil and a decrease in lymphocyte count occurs during stress [10,18]. Because stress stimulates the anterior pituitary gland to secrete adrenocorticotropic hormone which induces the adrenal cortex to produce glucocorticoids, involved in the mobilization of neutrophils into the peripheral circulation [18]. An increase in neutrophils will cause a decrease in lymphocytes. Neutrophils were significantly negatively correlated with lymphocytes (r = −0.95) [19].
Haematological traits are essential parameters for evaluating the health and physiological status of animals that can reflect the physiological responsiveness [7]. Haematological parameters have been commonly used as indicators of pre-slaughter stress [10]. Pre-slaughter stress is the main factor affecting meat quality [5] due to a faster muscle glycogen breakdown [6]. High pre-slaughter stress was related to increased drip loss in pork [20]. This study indicated that pork with high water-holding capacity showed higher WBC and monocyte count. Previous study indicated that animals with high WBC have enhanced adaptability to environmental stresses [8]. Also, normal pigs had higher WBC, lymphocytes, and monocytes in comparison to pigs with a chronic stress syndrome due to failure to adapt to the new environmental [21]. In addition, WBC was positive correlated with monocytes (r = 0.58) [19]. However, it is unclear regarding haematological changes to the physiological response. Previous study has reported that the increasing in WBC may indicate that cell damage occurred during the pre-slaughter period. This resulted in an inflammation response which in turn led to an increase in the amount of WBC in the blood [9].
Muscle pH
This study indicated that pork with lower drip loss showed higher muscle pH at 45 min and 24 h post-mortem than pork with higher drip loss. The previous study reported that drip loss was a negatively correlated to pH at 45 min and 24 h postmortem (r = −0.40 and −0.50, respectively) [22]. Muscle pH is the best indicator of muscle to meat conversion including meat color, texture and moisture [5]. Once the muscle has shifted to use anaerobic glycolysis as a major energy-generating pathway, the pH is reflective of the accumulation of lactic acid within the muscle, resulting in the pH decline in post-mortem muscle. The muscle pH early post-mortem (45 min) has been used to monitor the quality difference in fresh pork [3]. Drip loss varies due to post-mortem metabolism as a result of ATP degradation and the rate of acidification [4]. The faster pH decline caused denaturation of sarcoplasmic and myofibrillar proteins, resulting in reduced water holding capacity [23]. Furthermore, when the pH reaches the isoelectric point (pI) of the major proteins (pH = 5.4), the result is a reduced amount of water that can be attracted and held by the protein [24], and a reduced repulsion of structures within the myofibril. Water moves from the myofibril into the extramyofibrillar spaces, where it eventually is lost from the muscle cell [25].
Serum cortisol levels
Pre-slaughter stress stimulates the two main stress-responsive neuroendocrine systems including the HPA axis and the sympathetic nervous system. The activation of the HPA axis regulates secretion of cortisol in response to stress [26]. Pigs handled with high stress at pre-slaughter had higher levels of cortisol compared with low stress [27]. Serum cortisol levels increased during the journey (3.47 μg/dL at loading and 8.52 μg/dL at unloading) but decreased during the lairage (6.96 μg/dL at exsanguination) [13]. Increased levels of serum cortisol related to increased blood glucose and lactate level, resulting in a faster muscle pH decline, high drip loss, and lightness in pork [16]. Previous studies have investigated the association between cortisol level and meat quality [12,16]. This study shown serum cortisol level did not differ between drip loss groups. The same result as the previous reports, as the level of cortisol was not associated with meat quality [15,28]. This is explained by the fact that the plasma clearance of cortisol is rapid [29]. In pigs, the cortisol levels rose immediately after the start of transport and decreased rapidly after unloading [30]. Of all stress indicators (lactate, cortisol, and catecholamines) measured at exsanguination, only blood lactate was strongly correlated with pork quality traits [20]. Therefore, this result indicates that measurement of serum cortisol levels may not be a good indicator for meat quality in pigs.
In conclusion, the present study indicated that the main factor affecting drip loss is the pH decline in post-mortem muscle. The low drip loss group had a higher muscle pH at 45 min and 24 h post-mortem than the high drip loss group. Moreover, blood haematological parameters including WBC and monocyte count affected drip loss. The low drip loss group had higher WBC and monocyte count than the high drip loss group. There was no effect of the serum cortisol levels on drip loss. Serum cortisol levels might be not the potential indicator parameters for drip loss in pork.
ACKNOWLEDGMENTS
The authors are grateful to Department of Animal Science and Central Laboratory, Faculty of Agriculture, Kasetsart University for the utilization of laboratory facilities.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.