Effects of β-Glucan on the Release of Nitric Oxide by Macrophages Stimulated with Lipopolysaccharide
Article information
Abstract
This research analyzed the effect of β-glucan that is expected to alleviate the production of the inflammatory mediator in macrophagocytes, which are processed by the lipopolysaccharide (LPS) of Escherichia. The incubated layer was used for a nitric oxide (NO) analysis. The DNA-binding activation of the small unit of nuclear factor-κB was measured using the enzyme-linked immunosorbent assay-based kit. In the RAW264.7 cells that were vitalized by Escherichia coli (E. coli) LPS, the β-glucan inhibited both the combatant and rendering phases of the inducible NO synthase (iNOS)-derived NO. β-Glucan increased the expression of the heme oxygenase-1 (HO-1) in the cells that were stimulated by E. coli LPS, and the HO-1 activation was inhibited by the tin protoporphyrin IX (SnPP). This shows that the NO production induced by LPS is related to the inhibition effect of β-glucan. The phosphorylation of c-Jun N-terminal kinases (JNK) and the p38 induced by the LPS were not influenced by the β-glucan, and the inhibitory κB-α (IκB-α) decomposition was not influenced either. Instead, β-glucan remarkably inhibited the phosphorylation of the signal transducer and activator of transcription-1 (STAT1) that was induced by the E. coli LPS. Overall, the β-glucan inhibited the production of NO in macrophagocytes that was vitalized by the E .coli LPS through the HO-1 induction and the STAT1 pathways inhibition in this research. As the host immune response control by β-glucan weakens the progress of the inflammatory disease, β-glucan can be used as an effective immunomodulator.
INTRODUCTION
The inflammation is a mechanism that recovers or reproduces the damaged tissues, as the immune cells secrete the various inflammation mediators after they perceive the physical and chemical stimulations or the virus infections from the outside. However, a chronic inflammation that occurs continuously or excessively causes a damage to the tissues, and the active oxygen species and the inflammatory cytokine that are related to this play the important roles as the media of the various diseases, including the endotoxin stimulations (Chung et al., 2011). Macrophagocyte is known to help maintain the homeostasis by being involved in not only the innate immunity, but, also, the various host reactions, including the acquired immunity. When an inflammation occurs, these inflammatory cells, such as macrophagocyte, cause pain, edema, and heat and facilitate the movement to the immune cells to the inflamed parts by forming nitric oxide (NO) and prostaglandin E2. As a typical example of inflammation, macrophagocyte recognizes lipopolysaccharide (LPS), one of the inflammatory mediators, as the formation of the heterodimer of the toll-like receptor 4 and induces the activation of the nuclear factor-κB (NF-κB), which is a transcription factor within the cells (Shon et al., 2012). NF-κB, which has moved to the nucleus, induces the gene expression of inflammatory cytokine, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2), while NO, the indicator of an inflammation is synthesized by NO synthase (NOS) from L-arginine (Shon et al., 2012). Three similar forms of NOS exist, including neuronal NOS (nNOS), endothelial NOS (eNOS), and iNOS. Among them, the NO formation by the iNOS, in particular, plays an important role pathologically (Kim et al., 2011). While nNOS and eNOS are always expressed, the expression of iNOS is induced by LPS or inflammatory cytokine, and the NO that is excessively formed by iNOS intensifies the inflammation reaction and causes the damage to the tissues (McCartney-Francis et al., 1993).
β-Glucan is known to exist inside the cell walls of grain, mushroom, and yeast. It is known to dramatically enhance the immunity function in the body when it is absorbed into the digestive organs, and efficiently inhibit and eliminate harmful microorganisms, viruses, toxins, and fungus through the various stimulations. In addition, β-glucan strengthens the function of the macrophagocyte inside the immune system, thereby causing the macrophagocyte to secrete cytokine—the growth factor of other lymphocytes or white blood cells—and reinforce the function of the entire immune system. As a result, this helps prevent the various adult diseases and cancer. Also, the β-glucan intake contributes to the strengthening of the immunity of the human and animal body by promoting the growth of the intestinal bacterial flora (Compton et al., 1996). For the biological activation of β-glucan, the anti-tumor effect, the defense effect against virus attack, and the injury-healing and improvement effect have been reported in in vivo (Onderdonk et al., 1992; Ohno et al., 1995). There are reports that, in in vitro experiments, β-glucan changes the form of the macrophagocyte and influences the production of the cytokine, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 or IL-1, the production of NO, the secretion of lysosomal enzyme, the production of hydrogen, the metabolism of arachidonic acid, and the alternative pathway of the complement (Ohno et al., 1995; Thornton et al., 1996). It has been reported that β-glucan activates macrophagocyte through a macrophagocyte-specific acceptor or is combined with the complement CR3 (Di et al., 1991; Thornton et al., 1996). Especially, Aureobasidium pullulans, which is a black yeast, is widely used in the industrial production by fermentation. A. pullulans produces the receptive β-glucan, which consists of the main β-(1,3)-linked glucose chain and the β-(1,6)-linked glucose branches, outside of the cells in a certain growth condition (Hamada et al., 2000). The A. pullulans incubation fluid containing β-glucan as the main ingredient is consumed as a supplementary food in many countries as it represents the activation of the immune-stimulation. Also, A. pullulans incubation fluid is regarded as useful and effective for delaying the outbreaks of many diseases, and has been reported to show the activation of anti-tumor (Kimura et al., 2006), anti-allergy (Kimura et al., 2007), and anti-inflammation (Muramatsu et al., 2014) in the mouse model.
This research aims to determine the anti- inflammatory effect of A. pullulans β-glucan. It examined the change in the amount of the NO that is released by the RAW 264.7 cells, of which the inflammation is induced by LPS and measured the anti-inflammatory function and its level in order to analyze the usefulness of β-glucan as an immunomodulatory.
MATERIALS AND METHODS
Reagents
The test materials, including β-glucan, were supplied from Glucan Corp., Busan, Korea, which obtained them through the fermentation of Aureobasidum pullans SM-2001. According to the previous study (Seo et al., 2002), the major compounds are known to be polysaccharides, including 15% of β-1,3/1,6-glucan. The first antibody for iNOS, HO-1, NF-κB p65, NF-κB p50, poly(ADP-ribose) polymerase-1 (PARP-1), and β-actin were purchased at Santa Cruz Biotechnology (Santa Cruz, CA, USA), while the first antibody of c-Jun N-terminal kinases (JNK), phospho-JNK, p38, phospho-p38, extracellular signal-regulated kinases (ERK), phospho-ERK, inhibitory κB-α (IκB-α), signal transducer and activator of transcription-1 (STAT1) and phospho-STAT1 was purchased at Cell Signaling Technology (Beverly, MA, USA). Next, tin protoporphyrin IX (SnPP) was purchased at Frontier Scientific Inc. (Logan, UT, USA), and other chemical reagents were purchased at Sigma-Aldrich (St. Louis, MO, USA).
Cell incubate
The mouse macrophage cell line of RAW 264.7 was used for the subincubate once every 3 days by using Dulbecco’s modified Eagle’s medium that was prepared by adding a 10% [v/v] heat-inactivated fetal bovine serum, 100 unit/mL penicillin and 100 μg/mL streptomycin in a 5% CO2 incubator at a temperature of 37°C. The cells were distributed to the plates at a concentration of 1×106 cells/mL and incubated for more than 2 h to attach the cells onto the plates. LPS (1 μg/mL) was treated with β-glucan and incubated for 24 h (Choi et al., 2007).
Cytotoxicity assay
The cytotoxicity of β-glucan against the RAW 264.7 cells was tested using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The assay used the conversion of MTT into a formazan through the action of mitochondrial dehydrogenases. MTT (5 mg/mL) was dissolved in the phosphate-buffered saline (PBS) and filtered using a 0.2 μm syringe filter, and then stored at 2°C to 8°C for the analyses. LPS (1 μg/mL) and the various concentrations of β-glucan were added to the cells, which was then incubated for 24 h. After the incubation, the solution was removed and MTT was added to reach the final concentration of 0.5 mg/mL and then it was incubated for 3 h at 37°C in a 5% CO2 incubator. After the incubation, the supernatant was removed and dimethyl sulfoxide was added to dissolve the cells, and the absorbance of the formed formazan was measured at a wavelength of 570 nm using a Spectra Max 250 ELISA Reader (Molecular Devices, Sunnyvale, CA, USA). The cell viability was evaluated in percentage by comparing it with the control group (Choi et al., 2011).
Measurement of NO production
From the LPS-activated RAW 264.7 cells, the nitrite (NO2−) concentration of the incubated supernatant was measured using the Griess analysis technique to analyze the inhibition of the NO production by β-glucan. To stabilize the cells after distributing 5×105 cells/well to the 24-well flat-bottomed microtiter plates, the plates were incubated for a day at 37°C in a 5% CO2 incubator. After the incubation, β-glucan was added to the cell incubate and incubated for 24 h at 37°C in a 5% CO2 incubator. Then, the sodium nitrite concentration at the supernatant was measured by using the Griess reagent. By using a 96 well plate, 100 μL of the Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, and 2.5% phosphoric acid) (sigma), which was equivalent to the volume of the cell incubate supernatant, was mixed and reacted for 10 min to measure the absorbance at the wavelength of 540 nm using a Spectra Max 250 ELISA Reader (Molecular Devices, USA). The nitrite concentration was determined by using a standard curve that was produced by consecutively diluting NaNO2 (sigma) with the incubate solution (Choi et al., 2007).
RNA extraction and real-time polymerase chain reaction
Cells were seeded in 60 mm tissue incubate dishes at a density of 4×106 cells/dish and incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS. The cell incubates that were incubated with the β-glucan were washed 3 times with PBS. For the separation of the total RNA in the cells, the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) was used for the separation by following the manufacturer’s guidelines. The concentration of the separated RNA was determined by measuring the absorbance at 260 nm. By using the iScript cDNA Synthesis Kit (BIO-RAD/170-8891), the cDNA was synthesized from the separated RNA, and the real time polymerase chain reaction (PCR) was performed by using the SsoFast EvaGreen Supermix (BIO-RAD/172-5201) and the thermal cycler (CFX 96 Real Time PCR, Bio-Rad, Hercules, CA, USA). The PCR amplification condition was set to perform 1 cycle at 98°C for 30 s, which was followed by 45 cycles of the reactions at 95°C for 1 s and 60°C for 5 s. As the internal control, β-actin was used. The specific oligonucleotide primer that was designed to amplify the iNOS cDNA is listed below: iNOS, 5′-GCA CCA CCC TCC TCG TTC AG-3′ (sense), 5′-TCC ACA ACT CGC TCC AAG ATT CC-3′ (antisense); β-actin, 5′-TGA GAG GGA AAT CGT GCG TGA C-3′ (sense), 5′-GCT CGT TGC CAA TAG TGA TGA CC-3′ (antisense) (Choi et al., 2012).
Immunoblot analysis
Cells were seeded in 60 mm tissue incubate dishes at a density of 4×106 cells/dish and incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS. Nuclear extracts were prepared using the nuclear extract kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions. To prepare the whole-cell protein extracts, the cells were lysed with the sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% w/v SDS, 10% glycerol, 50 mM DTT and 0.01% w/v bromophenol blue). The lysed sample solution was sonicated for 10–15 sec to remove the viscosity by using an ultrasonicator, and the whole cell lysate was acquired by boiling at 100°C for 5 min. The proteins within the cell lysates (30 μg) were separated using a 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred to a nitrocellulose paper. After the membrane blocking treatment was performed for 1 h with 5% skim milk that was prepared by dissolving at PBS-Tween-20, it was reacted with the primary antibody. After that, it was washed 3 times with the PBS-Tween-20 buffer and reacted with the horseradish peroxidase-conjugated secondary antibody, which was used to observe the antibody-specific protein by using an enhanced chemiluminescence detection system (ECL) (Amersham Pharmacia Biotech, USA) (Choi et al., 2012).
DNA-binding activities of NF-κB subunits
Cells were plated in 60 mm tissue incubate dishes at a density of 4×106 cells/dish and incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/ml) for the different periods of time. After preparing the nuclear extracts from the cells as described above, the DNA-binding activities of NF-κB p65 or p50 in the nuclear extracts were quantified using the ELISA-based NF-κB transcription factor assay kit (Active Motif) according to the manufacturer’s instructions (Choi et al., 2011).
Statistical analysis
All experiments were repeated 3 times, and the results were analyzed by using the student’s paired t-test. The analysis was considered to be statistically significant when p<0.05.
RESULTS
Effect of β-glucan on NO formation and cytotoxicity
The anti-inflammatory effect of β-glucan was examined by measuring the amount of the NO formation in the RAW 264.7 cells, which is a mouse macrophage cell line. Compared to the very little amount of NO formed in the control group, the amount of NO in the cell that processed Escherichia coli (E. coli) LPS (1 μg/mL) increased remarkably. The amount of the induced NO was decreased noticeably by β-glucan as it was concentration-dependent, and, when 600 μg/mL of β-glucan was added, the NO formation was inhibited by about 70%. As the concentration of the β-glucan increased, the amount of the NO formation decreased and showed the inflammatory-inhibitive effect, which was statistically significantly declined (Figure 1A). In order to check whether the decrease of the NO formation was due to the extinction of the cells by β-glucan in the experimental group, the control group and experimental group were incubated for 24 h and the MTT assay was carried out. The result was that, while the concentration of β-glucan increased in the experimental group, there was no difference in the survival of the cells compared to the control group (Figure 1B).

Effects of β-glucan on Escherichia coli (E. coli) lipopolysaccharide (LPS)-induced production of NO2− and on viability in RAW264.7 cells. (A) The cells were indicated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/mL) for 24 h, after which the levels of nitric oxide (NO) in the incubate media were determined. (B) Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The results are means±standard error of the mean. of three independent experiments. * p<0.05 versus E. coli LPS alone; ** p<0.01 vs E. coli LPS alone.
Effect of β-glucan on iNOS protein and mRNA expression
In order to check whether the inhibition effect of β-glucan on the NO formation is through the inhibition of the iNOS protein expression, the effect on the iNOS protein expression by E. coli LPS was assessed. The experiment result demonstrated that the control group where E. coli LPS was not added and that was not activated did not have the expression of the iNOS protein, but, the expression of about 130 kDa protein, which reacts with iNOS-specific antibody, was observed in the experimental group that was activated by 1 μg/mL of E. coli LPS. In the experimental group where β-glucan was added, the expression of the iNOS protein by E. coli LPS was inhibited concentration-dependently. Thus, it was observed that, as the concentration of β-glucan increased, the expression of the iNOS protein decreased in the same aspect regarding the inhibition of the NO formation (Figure 2). The effect of β-glucan on the iNOS transcription and the expression of iNOS mRNA by E. coli LPS was verified through the real-time PCR. When the RAW 264.7 cells were exposed to 1μg/mL of E. coli LPS, iNOS mRNA was expressed. However, β-glucan inhibited the expression of iNOS mRNA in macrophagocyte by E. coli LPS, thereby adding 600 μg/ml of β-glucan which inhibited the expression iNOS mRNA by about 50% (Figure 3).
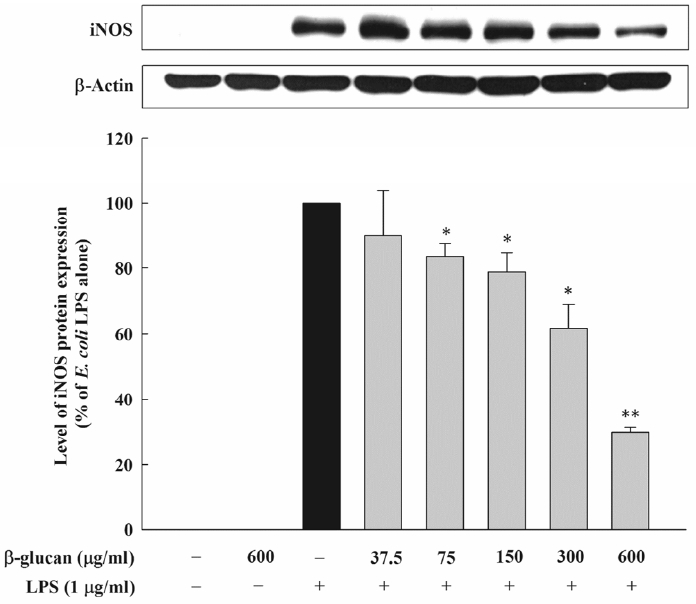
Effect of β-glucan on iNOS protein expression induced by Escherichia coli LPS in RAW264.7cells. Cells were incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/mL) for 24 h, after which iNOS protein synthesis was determined by immunoblot analysis of cell lysates using iNOS-specific antibody. A representative immunoblot from three separate experiments with similar results is shown. iNOS, inducible nitric oxide synthase.

Effects of β-glucan on iNOS mRNA expression induced by Escherichia coli LPS in RAW264.7 cells. Cells were incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/mL) for 24 h, after which real-time PCR was performed with EvaGreen Supermix. iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide.
Effect of β-glucan on HO-1 induction
An immunoblot was performed to examine whether the HO-1 that was induced by β-glucan would control the expressions of the NO and the HO-1 that were mediated by E. coli LPS. Cells were incubated with various concentrations of β-glucan (0, 37.5, 75, 150, 300, and 600 μg/mL) in the absence or presence of E. coli LPS (1 μg/mL) for 6 h, and the induction of the HO-1 protein was determined by an immunoblot analysis of the cell lysates using a specific antibody against HO-1. β-glucan dramatically increased the expression of HO-1 that was concentration-dependent in the cell that processed E. coli LPS (Figure 4A). Since β-glucan induced the HO-1 expression in the cell that processed E. coli LPS, the experiment was carried out after it was judged that the inhibition effect of β-glucan on the formation of the LPS-induced NO would be related to the HO-1 induction. The cell that processed β-glucan and E. coli LPS (1 μg/mL) processed SnPP, a competitive HO-1 inhibitor, through concentration. After incubating it for 24 h, the amount of the NO formation was measured on the incubated layer. This demonstrated that SnPP was concentration-dependent and hindered β-glucan’s NO formation inhibition (Figure 4B).

Involvement of HO-1 in the inhibitory effects of β-glucan on NO production in RAW264.7 cells activated by Escherichia coli LPS. (A) Cells were incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/mL) for 6 h. HO-1 protein synthesis was determined by immunoblot analysis of cell lysates using HO-1 specific antibody. A representative immunoblot from three separate experiments with similar results is shown. (B) Cells were incubated with β-glucan (600 μg/mL) and E. coli LPS (1 μg/mL) for 24 h in the presence of different doses of SnPP, after which incubate supernatants were assayed for NO. The results are means±SEM of three independent experiments. ** p<0.01 versus E. coli LPS alone; # p<0.05 versus E. coli LPS plus β-glucan. HO-1, heme oxygenase-1; NO, nitric oxide; LPS, lipopolysaccharide; SEM, standard error of the mean.
Effect of β-glucan on MAPK phosphorylation
It is known that MAPK, NF-κB, and the Janus kinase (JAK)2/STAT1 pathway are involved in the NO formation by LPS. Thus, this research examined whether β-glucan inhibited the formation of the E. coli LPS-induced NO through the MAPK pathway control. Through E. coli LPS stimulation, MAPK were phosphorylated. In contrast, β-glucan could not inhibit the phosphorylation of the MAPK that were activated by LPS (Figure 5). These results indicate that the MAPK pathway is not involved in the E. coli LPS- induced NO formation by β-glucan.
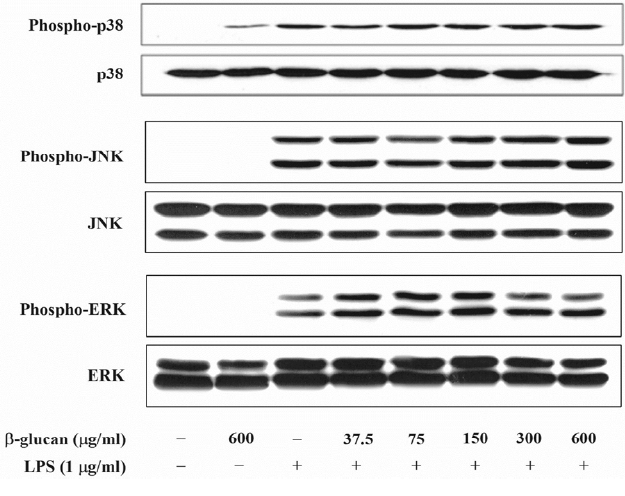
Effects of β-glucan on the phosphorylation of MAPK induced by Escherichia coli LPS in RAW264.7cells. Cells were incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/mL) for 30 min, after which cells lysates were subjected to immunoblot analysis using specific antibodies. A representative immunoblot from three separate experiments with similar results is shown. LPS, lipopolysaccharide.
Effect of β-glucan on NF-κB activation
IκB-α is known as the controller of the NF-κB activity, and it is controlled by forming a homo-dimer and a heterodimer with the NF-κB in the cytoplasm. IκB-α is combined with NF-κB p65 and p50, and, when there is the NF-κB stimulation, it is degraded through the phosphorylation process, and it controls the NF-κB activation. This research analyzed the effect of β-glucan on the signaling pathway of NF-κB, which mediates the production of E. coli LPS-induced NO. In order to verify the effect of β-glucan on the degradation of IκB-α, which is the upstream signaling pathway of E. coli LPS-induced NF-κB, the level of the cytoplasm of the IκB-α protein was measured through an immunoblot analysis. As expected, the degradation of IκB-α was noticeably observed in the cell that processed E. coli LPS. However, β-glucan could not inhibit the degradation of E. coli- induced IκB-α (Figure 6A). Next, this research examined the effect of β-glucan on the nuclear translocation of NF-κB p65 and p50, which are the downstream of the IκB-α degradation. An immunoblot analysis was carried out by preparing nuclear fractions and by using an antibody against NF-κB p65 and p50. PARP-1 was used as an internal control and E. coli LPS remarkably induced the nuclear translocation of the NF-κB subunits. The maximum expression time of NF-κB p65 was discovered 30 min after adding LPS, and the maximum expression time of NF-κB p50 was discovered 8 h after adding LPS (data not presented). However, β-glucan did not have any influence on the nuclear translocation of NF-κB p65 and p50 that were induced by E. coli LPS (Figure 6B). Finally, this research checked whether β-glucan could influence the NF-κB-dependent transcription by inhibiting the combination of DNA and NF-κB. The DNA-bound activity of NF-κB inside the nucleus was analyzed using the ELISA-based NF-κB p65/NF-κB p50 transcription factor assay kits, which showed that the DNA-bound activity of NF-κB p65 and p50 increased dramatically by E. coli LPS. On the contrary, the DNA-bound activity of NF-κB p65 and p50 that were induced by E. coli LPS was not inhibited by β-glucan (Figure 6C).
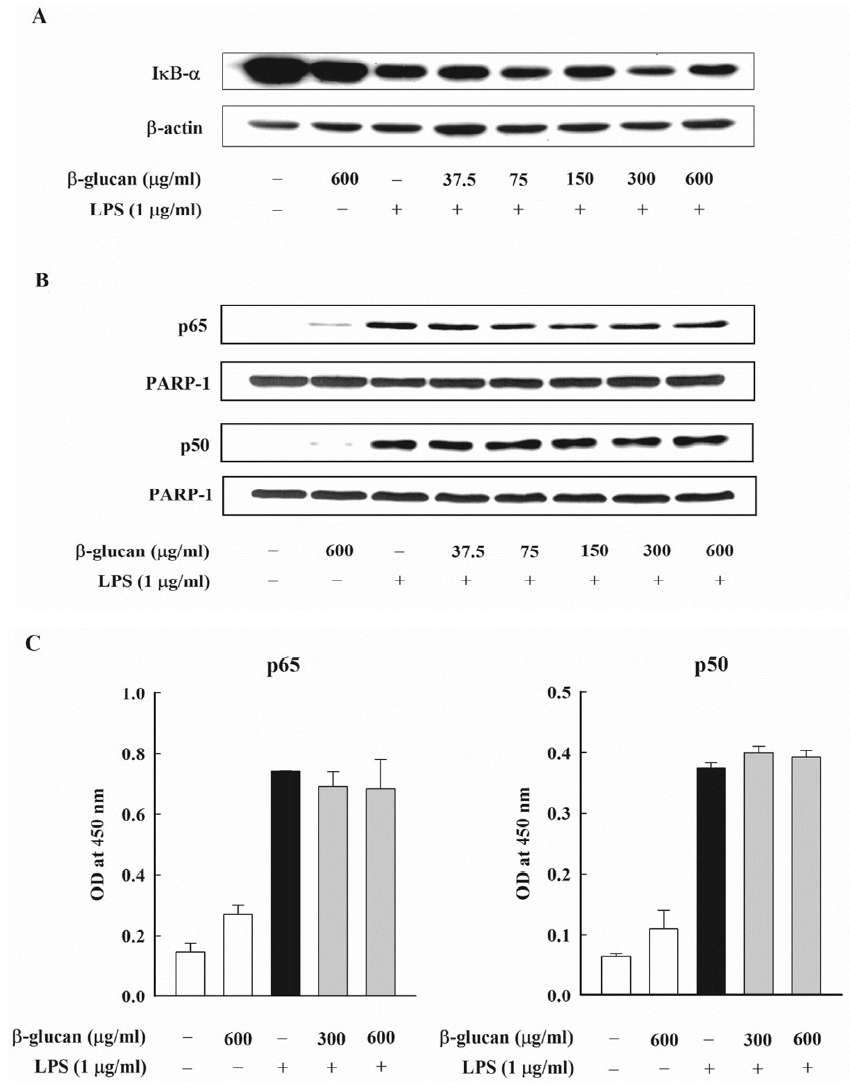
Effects of β-glucan on activation of NF-κB induced by Escherichia coli LPS in RAW264.7cells. (A, B) Cells were incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/mL). (A) After 30 min of incubation, IκB-α degradation was determined by immunoblot analysis of cell lysates using antibody. A representative immunoblot from two separate experiments with similar results is shown. (B) After 30 min (for NF-κB p65) or 8 h (for NF-κB p50) of incubation, the nuclear fraction was isolated from cells. Nuclear translocation of NF-κB subunits was analyzed by immunoblot analysis using antibodies against NF-κB p65 and p50. A representative immunoblot from two separate experiments with similar results is shown. (C) Cells were incubated with β-glucan (300 and 600 μg/mL) in the absence or presence of E. coli LPS (1 μg/mL). After 30 min (for NF-κB p65) or 8 h (for NF-κB p50) of incubation, the nuclear fraction was isolated from cells. DNA-binding activities of NF-κB subunits in nuclear fraction was assessed by using the ELISA based NF-κB p65/NF-κB p50 transcription factor assay kits. NF-κB, nuclear factor- κB; LPS, lipopolysaccharide; IκB-α, inhibitory κB-α; ELISA, enzyme linked immunosorbent assay.
Effect of β-glucan on STAT1 phosphorylation
The JAK2/STAT1 pathway is known to be involved in the NO formation by LPS. Therefore, this research examined the inhibition regarding the formation of the E. coli LPS-induced NO by β-glucan through the JAK2/STAT1 signaling pathway control. An immunoblot was performed by preparing cell lysates and by using the antibody against STAT1 and phosphor-STAT1. As a result, β-glucan noticeably inhibited the phosphorylation of STAT1 that was induced by E. coli (Figure 7).
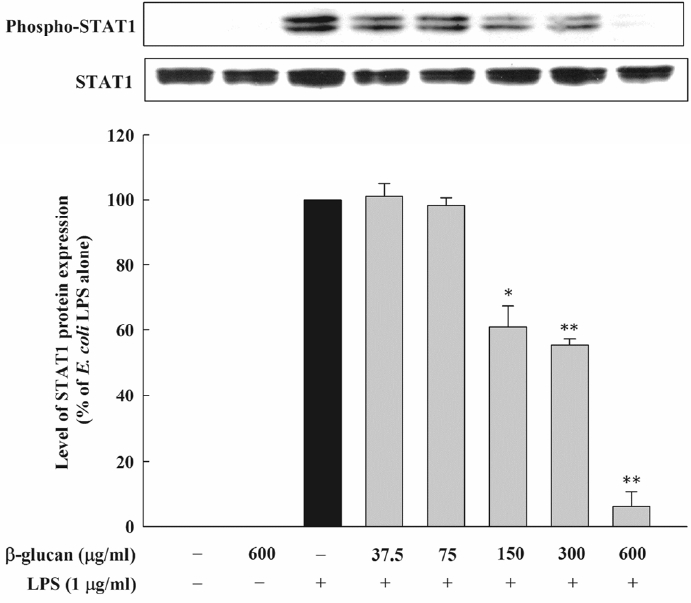
Effect of β-glucan on the phosphorylation of STAT1 induced by Escherichia coli LPS in RAW264.7 cells. Cells were incubated with various concentrations of β-glucan in the absence or presence of E. coli LPS (1 μg/mL) for 4 h, after which phosphorylation of STAT1 was determined by immunoblot analysis of cell lysates. Are presentative immunoblot from two separate experiments with similar results is shown. STAT1, signal transducer and activator of transcription-1; LPS, lipopolysaccharide.
DISCUSSION
The inflammation reactions that appear in relation to the allergic diseases are the non-specific immunity reactions of the biological tissues regarding the stimulations. They are the complicated lesions that cause the degenerations of the tissues (Morson, 1980). Also, macrophagocyte is a typical immune cell that is involved in early inflammation reaction; it exists in all tissues of the animal body and plays a very important role with regard to the various inflammatory diseases by detecting foreign substances from outside and it eliminates dead cells through phagocytosis and through the formations of cytokine and protein, which induces an inflammation such as TNF-α, IL-6, COX-2, iNOS, through external stimulation (Chae et al., 2009). It has been known that the increase of the substances, such as NO, that induce inflammation is related to causing inflammatory diseases, cardiovascular disease, cancer, and diabetes, and it has also been reported that NO plays an important mediating role in inflammatory reaction (Nathan and Hibbs, 1991). NO, which is formed as a byproduct in the changing process of L-arginine into L-citrulline, is an important substance for maintaining the homeostasis of cells and is synthesized in many different types of cells with various stimulations. It is known to act positively in body functions such as vasodilation, neurotransmission system, antimicrobial substance, and immune modulation (Weisz et al., 1996). However, NO that is excessively increased by pathogenic organisms causes the destruction of cells and inflammation reactions (Min et al., 2010). In addition, the increase of the formation of NO by iNOS has been reported to be the causes of various diseases, which has led to conducting the researches on the methods that can selectively inhibit the activation of iNOS and which reduce the formation of cytokine that induces inflammation (Chiou et al., 2000). Therefore, anti-inflammation effects can be identified through the inhibition of the substances that were formed during such inflammation reaction processes.
Differently from the β-glucan which have been researched until the present, this research used the black yeast β-glucan centered on β-1,3/1,6-glucan, which was developed by cultivating the transmutator within Aureobasidium at Glucan. The black yeast β-glucan that was developed is a complex body of biologically active ingredient that contains polysaccharide, protein, organic acid, or vitamins. In order to figure out the anti-inflammatory effect of β-glucan and its effectiveness as an immunomodulator, the NO elimination effect and mechanism of β-glucan were measured. As a result, with the density of β-glucan increasing, the creation of NO was markedly reduced. And, with the reduction of the expressions of iNOS protein and mRNA, and, through the suppression of the NO creation and the similar aspects, the inflammation suppression effect appeared. β-Glucan suppressed approximately 70% of the NO creation amount that has been induced according to E. coli LPS. And the expression of the iNOS protein, too, was suppressed by approximately 70%. However, the amount of the expression of iNOS mRNA was suppressed with a small amount of approximately 50% differently from NO creations and the ratio of the suppression of the iNOS protein expression. Based on such results, it is considered that β-glucan more deeply participates in the posttranscriptional phase during the NO creation process.
HO-1 has various potentially beneficial effects such as anti-cancer, anti-inflammatory, and antioxidant activation (Morse and Choi, 2002; Otterbein et al., 2003; Ryter et al., 2006). A severe inflammation is observed in an HO-1-deficient mouse, while the excessive expression of HO-1 dramatically decreases the inflammation (Otterbein et al., 2003). This research tested the possibility that the inhibition effect of β-glucan on the production of the LPS-induced NO is related to the induction of HO-1. β-Glucan increases HO-1 expression by being concentration-dependent in the murine macrophagocyte that is stimulated by E. coli LPS, and the SnPP processing significantly reversed the inhibition effect of β-glucan in the production of the LPS-induced NO. This shows that the HO-1 that is induced by the β-glucan processing mediates the inhibition of the NO formation in the cells that are activated by LPS.
MAPKs, which include ERK, JNK, and p38, are the components that are important to the many intracellular signaling pathways and the activation of phosphorylation. The diverse members of the MAPK family play the important roles with regard to the inflammation reactions and the creation of the inflammatory media substances of the macrophages (Carter et al., 1999). As a result, we conducted a research on whether the NO creation suppression effects of β-glucan have any relation with the suppression of the vitalization of MAPKs. In this research, E. coli LPS stimulated the vitalizations of the three MAPKs of ERK, JNK, and p38 in the RAW264.7 cells. However, because β-glucan could not suppress the phosphorylation of MAPKs that has been vitalized due to E. coli LPS, we can know that the MAPK pathway does not play an important role with regard to the suppression of the NO creations by β-glucan.
NF-κB is a well-established regulator of inflammatory gene expression and LPS is one of the known provokers of the NF-κB activity (Karin and Ben-Neriah, 2000). In unstimulated conditions, NF-κB is sequestered in the cytoplasm in a latent form linked to the inhibitory proteins, called “the inhibitory κB (IκB) proteins”. When stimulated with a multiplicity of stimuli, including LPS, IκB becomes phosphorylated and ubiquitinated, followed by the consequent proteasome-mediated degradation. The free NF-κB is translocated into the nucleus and causes the transcription of the genes related with the inflammatory response (Covert et al., 2005). We carried out a research on the effects of β-glucan on the NF-κB signal delivery paths that mediate the NO production. However, β-glucan did not have any influences on the degradation of IκB-α, which was induced by E. coli LPS. And it did not have any influences on the nuclear translocations of the NF-κB p65 and p50 subunits. Also, by not having any influences on the vitalization of the DNA bindings on p65 and p50, which were induced by LPS, it did not get involved in the transcriptional activation that relies on NF-κB. Generally, with regard to the inflammation reactions, the NF-κB pathway plays an important role representatively. Hence, for more accurate results, there is a need for us to conduct additional experiments.
The other important transcription factors that play important roles in controlling the inflammation reaction that is induced by LPS are the STAT. Among seven mammalian STAT family members, STAT1 and STAT3 are the major controllers expressing the various inflammation mediators in the macrophagocyte that is activated by LPS (Gao et al., 1998; Samavati et al., 2009). It has been discovered that β-glucan inhibits the secretion of the NO in the RAW264.7 cells that were activated by E. coli LPS through the inhibition of the STAT1 signaling pathways. The mechanism through which E. coli LPS induces STAT1 phosphorylation and activation is related to the upstream kinase JAK2 of STATA1. JAK2 activates the downstream molecule STAT1 after the cells are stimulated by LPS and are activated (Schindler et al., 2007). The activated STAT1 is transferred into the nucleus, combines with a promoter-specific rank, and activates the transcription of the STAT1 reactive gene (Schindler et al., 2007). The mechanism of the β-glucan inhibiting STAT1 activation can be related to the inactivation of the upstream kinase JAK2, which mainly reacts to the activation of STAT1. The previous research demonstrated that HO-1 controls the expression of the LPS-induced iNOS by controlling the STAT1 expression in the RAW 264.7 cells (Tsoyi et al., 2008). Therefore, this research suggests that HO-1 induction is involved in the inhibition effect of β-glucan on the production of the E. coli LPS-induced NO through the STAT1 phosphorylation control (Wu et al., 2005).
Overall, this research indicates that β-glucan inhibits the production of the NO in the E. coli LPS-activated murine macrophages via the HO-1 induction and inhibits the STAT1 signaling pathways. Accordingly, it was concluded that the modulation of the host immune response by β-glucan would be an attractive therapeutic approach for attenuating the progression of inflammatory diseases. Furthermore, it is considered that β-glucan will be very useful for preventing or improving inflammatory diseases and will be very useful as immunomodulator. This research analyzed the effects of β-glucan in alleviating the production of the E. coli LPS-induced NO in the well-characterized RAW264.7 cells.
ACKNOWLEDGMENTS
This research was performed with the support of the Industry Core Technology Development Project (No. 10049026 and 10063302), Ministry of Trade, Industry, and Energy, Korea.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.