Effects of Supplementation of Eucalyptus (E. Camaldulensis) Leaf Meal on Feed Intake and Rumen Fermentation Efficiency in Swamp Buffaloes
Article information
Abstract
Four rumen fistulated swamp buffaloes were randomly assigned according to a 4×4 Latin square design to investigate the effects of Eucalyptus (E. Camaldulensis) leaf meal (ELM) supplementation as a rumen enhancer on feed intake and rumen fermentation characteristics. The dietary treatments were as follows: T1 = 0 g ELM/hd/d; T2 = 40 g ELM/hd/d; T3 = 80 g ELM/hd/d; T4 = 120 g ELM/hd/d, respectively. Experimental animals were kept in individual pens and concentrate was offered at 0.3% BW while rice straw was fed ad libitum. The results revealed that voluntary feed intake and digestion coefficients of nutrients were similar among treatments. Ruminal pH, temperature and blood urea nitrogen concentrations were not affected by ELM supplementation; however, ELM supplementation resulted in lower concentration of ruminal ammonia nitrogen. Total volatile fatty acids, propionate concentration increased with the increasing level of EML (p<0.05) while the proportion of acetate was decreased (p<0.05). Methane production was linearly decreased (p<0.05) with the increasing level of ELM supplementation. Protozoa count and proteolytic bacteria population were reduced (p<0.05) while fungal zoospores and total viable bacteria, amylolytic, cellulolytic bacteria were unchanged. In addition, nitrogen utilization and microbial protein synthesis tended to increase by the dietary treatments. Based on the present findings, it is suggested that ELM could modify the rumen fermentation and is potentially used as a rumen enhancer in methane mitigation and rumen fermentation efficiency.
INTRODUCTION
In ruminants, nutrients input are the first to fermentative digestion by ruminal microorganisms. Methane (CH4) production through enteric fermentation is of concern worldwide for its contribution to the accumulation of greenhouse gases in the atmosphere, as well as its being a waste of fed energy (Boadi et al., 2004). There is an interest in decreasing CH4 emission by inhibition of ruminal methanogens thus increasing the efficiency of feed energy utilization for ruminants and would have significant economic and environmental benefits (Guglielmelli et al., 2010; Benchaar and Greathead, 2011; Calabrò et al., 2013). Therefore, developing feeding strategies for ruminants with a methane suppressing impact are desirable and inhibition of methanogensis has long been considered as a strategy to improve animal productivity (Wanapat et al., 2013a,b).
Recently, animal nutritionists have paid more attention to manipulating rumen microbial ecosystems in order to reduce methane emission and N excretion by ruminants with the aim to improving feed conversion efficiency. Plant secondary metabolites, which are well documented as antimicrobial agents, are considered as potential candidates to achieve this objective, as they are available in large number and viewed as natural products that are safe for humans (Wanapat et al., 2013c; 2014). Eucalyptus is one of the world’s important and most widely planted genera. Among its main uses is the production of essential oils (EO) used for medicinal and pharmaceutical purposes. Essential oil from the leaves of Eucalyptus camaldulensis ranges from less than 1% to over 2%. As reported by Elaissi et al. (2012), eucalyptus contents several kinds of EO, the main components were 1,8-cineole (4.5% to 70.4%) followed by cryptone (0.0% to 20.9%), α-pinene (1.0% to 17.6%), ρ-cymene (0.8% to 16.7%), α-teprpineol (0.6% to 10.3%). Sallam et al. (2009, 2010) and Thao et al. (2014) proposed that eucalyptus leaves either fresh or as distilled eucalyptus oils have potential biological activities such as bacteriostatic, fungistatic, anti-inflommatory, modifying ruminal fermentation characteristics, anti-protozoal and methane mitigration. However, there are limited experimental data on effects of Eucalyptus leaves on rumen digestion and fermentation patterns, especially in swamp buffaloes. Therefore, the objective of this study was to evaluate the effects of Eucalyptus leaf meal (ELM) supplementation on feed intake and rumen fermentation characteristics of swamp buffaloes.
MATERIALS AND METHODS
Animal, feeds and management
Four, ruminal fistulated buffaloes, 4 years old with initial body weight (BW) of 321±20 kg, were randomly assigned to receive four dietary treatments according to a 4×4 Latin square design. The dietary treatments were based on different levels of ELM supplementation at 0, 40, 80, and 120 g/hd/d, respectively. Concentrate was fed daily at 0.3% BW and rice straw was offered ad libitum. The experiment was conducted for four periods and each lasted for 21 days. During the first 14 days, all animals were fed respective diets; whereas during the last 7 days, animals were moved to metabolism crates for total urine and fecal collection as well as rumen fluid and blood sampling.
Data collection and sampling procedures
Feeds and fecal samples were collected during the last 7 days of each period, dried at 60°C and ground to pass a 1-mm screen using a Cyclotech Mill (Tecator, Höganös, Sweden) and were analyzed using standard methods of AOAC (1995) for dry matter (DM), ash, and acid detergent fiber (ADF). Neutral detergent fiber (NDF) in samples was estimated according to Van Soest et al. (1991) with the addition of α-amilase but without sodium sulphite and the results were calculated with residual ash. Total nitrogen (N) of feeds, refusals and fecal samples were determined according to AOAC (1995).
At the last day of each period, rumen fluid and jugular blood samples were collected immediately post feeding at 2, 4, and 6 h. Rumen fluid was immediately measured for pH and temperature using a portable pH temperature meter. Ruminal ammonia nitrogen (NH3-N) was analyzed by micro Kjeltech Auto 1030 Analyzer (AOAC, 1995), volatile fatty acids (VFA) were analyzed by using high pressure liquid chromatography (HPLC) according to Samuel et al. (1997). Rumen fluid was also determined for direct count of protozoa and fungal zoospores using the methods described by Galyean (1989) by a haemacytometer (Boeco, Singapore) and bacteria (total viable, cellulolytic, amylolytic, and proteolytic) were measured using roll-tube technique (Hungate, 1969). A blood sample collected from the jugular vein at the same time as rumen fluid sampling was separated by centrifugation and stored at −20°C until analysis for blood urea nitrogen (BUN) according to Crocker (1967).
Urine samples were analyzed for total N (AOAC, 1995) and allantoin in urine was determined by HPLC as described by Chen et al. (1993). The amount of microbial purine derivatives (PD) absorption was calculated from PD excretion based on the relationship derived by the equation of Liang et al. (1994): Y= 0.12X+(0.02BW0.75). The supply of microbial N (MN) was estimated by urinary excretion of PD according to Chen and Gome (1995): MN (g/d) = 70X/(0.016×0.83×1,000) = 0.727X; where X and Y are, respectively, absorption and excretion of PD in mmol/d. Efficiency of microbial N synthesis (EMNS) was calculated using the following formula: EMNS = microbial N (g/d)/DOMR; where DOMR = digestible organic matter (OM) apparently fermented in the rumen (assuming that rumen digestion was 650 g/kg OM of digestion in total tract; DOMR = DOMI×0,65; DOMI = digestible organic matter in take).
Statistical analyses
All obtained data were subjected to analysis of variance according to a 4×4 Latin square design using the general linear models procedures of the Statistical Analysis System Institute (SAS, 1998). The results are presented as mean values with the standard error of the means. Differences among means with p<0.05 was accepted as representing statistical differences. Treatment means were compared by orthogonal polynomials by Duncan’s New Multiple Rang Test (Steel and Torrie, 1980).
RESULTS AND DISCUSSION
Chemical composition of diets
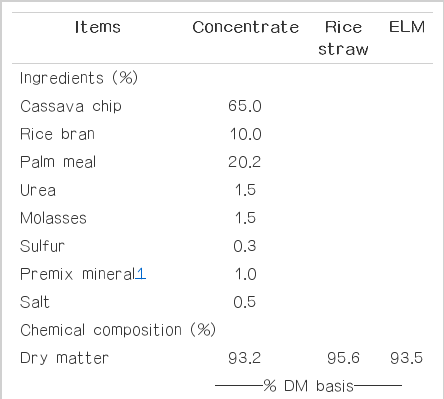
Table 1 shows feed ingredient and chemical compositions of concentrate, rice straw and ELM used during the four experimental periods. The concentrate consisting of available local feed resources such as cassava chip, rice bran, palm kernel meal, molasses, urea and minerals had a higher quality in terms of crude protein (CP) and low in NDF (14.2% and 16.0% DM, respectively), while rice straw contained a low level of CP but was high in NDF (3.2% and 76.2% DM, respectively) which was consistent with the study of Wanapat et al. (2013d). On the other hand, ELM contained CP up to 9.5% DM and was low in NDF and ADF (34.4% and 22.0% DM, respectively). Moreover, ELM contained tannin 9.0% DM. These values were similar to the finding of Manh et al. (2012). Sallam et al. (2010) who found that Eucalyptus fresh leaves had CP: 7.64%, NDF 61.62%, and ADF 50.4%. Moreover, Brooker and Kleinig (2006) suggested that the chemical composition of Eucalyptus and the individual concentration these chemicals varies with the species, season, location, climate, soil type, age of the leaves, fertility regime and the method used for drying the plant material.
Feed intake and nutrient digestibility
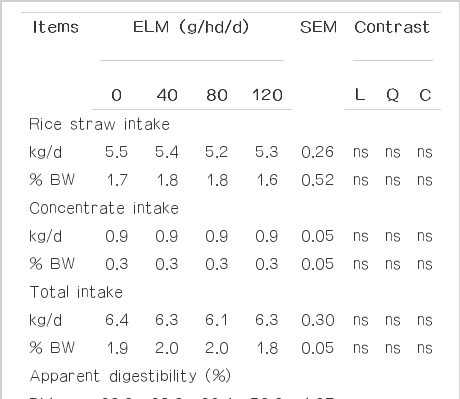
The effects of ELM supplementation on total dry mater intake and nutrient digestibility are presented in Table 2. Results of this study indicate that supplementing swamp buffaloes with ELM had no effect on feed intake. The effects of feeding essential oils (EO) or their components on feed intake have been variable depending on the type and dose of EO used (Patra et al., 2010; Thao et al., 2014). Reports on the effect of EO or their components on feed intake are very scarce because of the reduction of palatability (Salem et al., 2006). For instance, Cardozo et al. (2006) reported that supplementation of a mixture of cinnamaldehyde (0.6 g/d) and eugenol (0.3 g/d) oils decreased dry matter intake in dairy heifers. Furthermore, Benchaar et al. (2006) indicated that feeding dairy cows at 2 g/d of EO containing a mixture of thymol, eugenol, vanillin, and limonene decreased feed intake. In contrast, a low level of EO supplementation may stimulate feed intake. Similarly, testing a variety of EO including cinnamaldehyde, garlic, and juniper berry at 200 mg/kg of dietary DM fed to lambs, Chaves et al. (2008) did not find any effects on intake when the flavors were included in sheep diets. More recently, Giannenas et al. (2011) reported no change in DM in dairy ewes when supplemented with EO at 150 mg/kg of concentrate feed.
Apparent digestibility of DM, OM, CP, NDF, and ADF were not significantly different (p>0.05) among treatments in the present study. Moreover, Sallam et al. (2009) observed that supplementation of Eucalyptus oil did not affect on in vitro DM and OM digestibility. Furthermore, Santos et al. (2010) reported that the digestibility of feed was not affected when supplementation of EO complex to the diet of lactating dairy cows.
Rumen fermentation characteristics
Effects of ELM supplementation on rumen fermentation efficiency and BUN are presented in Table 3. Ruminal temperature, pH and BUN were similar among treatments and the values were stable at 38.5°C to 38.6°C, 6.4–6.6, and 9.3–9.4 mg/dL, respectively. However, the concentration of NH3-N was found the lowest in the treatment with 120 g ELM/h/d supplementation and the highest was in the control group. Castillejos et al. (2007) studied long term effect of EO on rumen fermentation in vivo also found that supplementation EO resulted in lower ruminal ammonia-N when compared to that in control. Several studies (Newbold et al., 2004; Patra and Saxena, 2009) demonstrated that EO might inhibit the hyper-ammonia producing bacteria in the rumen, which results in decreased amino acid deamination, consequently, lowering rumen NH3-N.
As shown, supplementation of ELM resulted in increasing total VFA concentration (p<0.05) and propionate molar concentration, while the molar proportion of acetate was decreased (p<0.05) with an increased level of ELM in the diets, concomitantly, calculated methane production was also decreased (p<0.05). The total VFA concentrations in the rumen were generally slightly affected (Chaves et al., 2008; Patra et al., 2010) or decreased especially at higher levels of supplemented EO. However, recent observed was similar with Chaves et al. (2008) and Wang et al. (2009) when supplementation of EO was given to sheep. The ELM supplementation caused a shift in end products of rumen fermentation with a reduction of acetate proportion and acetate : propionate ratio and was in agreement with several works in in vitro (Castillejos et al., 2006) and in vivo (Giannenas et al., 2011).
Calculated methane production based on molar VFA according to Moss et al. (2000) in present study showed that methane production was reduced and was in agreement with in vitro (Sallam et al., 2009) and in vivo (Wang et al., 2009). Modification of the fermentative profiles could be a result of inhibition of Archae (methanogens) and gram positive rumen microbes (i.e., acetate producers) as reviewed by Benchaar and Greathead (2011). In addition, this was accompanied with a reduction of protozoa count by ELM supplementation treatments, which could further explain the decreased methane production since ruminal protozoa provide a habitat for methanogens.
Rumen microbial population
Table 4 illustrates data on rumen microbes affected by ELM supplementation using direct count and roll tube technique. The present results show that total viable bacterial and fungal zoospores count were not changed in the supplemented diets. In contrast, proteolytic bacteria and protozoa were decreased in the ELM supplementation groups as compared with the control group. Several authors have suggested certain effects of EO compounds on specific bacteria populations. However, such effects of EO compounds on ruminal microbial populations are inconclusive (Wallace et al., 2008). For instance, Newbold et al. (2004) reported that ruminal protozoa counts were not affected when sheep and dairy cows were fed with 110 and 750 mg/d of a mixture of EO, respectively. Moreover, supplementation of dairy cows diets with 0.5 g of cinnamaldehyde per liter of rumen fluid had also no effect on the number of ciliate protozoa (Fraser et al., 2007). In contrast, Ando et al. (2003) observed that total number of protozoa was decreased when dairy steers were fed 200 g/d of peppermint.
Nitrogen utilization and microbial protein synthesis
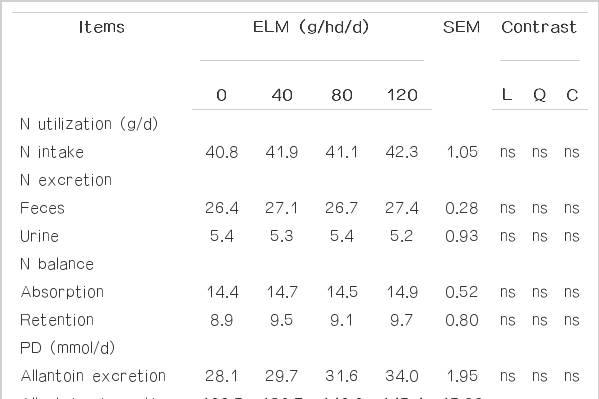
In buffaloes the effects of ELM supplementation in concentrate with rice straw as roughage on nitrogen metabolism and efficiency of microbial protein synthesis are summarized in Table 5. The results revealed that supplementation of ELM did not affect nitrogen utilization. In this study, it was observed that positive nitrogen retention and absorption were obtained in the ELM supplemented group and were not different among treatments. Similarly, Benchaar et al. (2006) observed no change in N retention when cows were fed with 2 g/d of the Crina ruminant supplement. According to Cutrignelli et al. (2007), PD excretion ranged between 28 and 34 mmml/d and was not affected by treatments. Moreover, no PD parameter was impacted by EML feeding; therefore calculated microbial protein synthesis yield also was not different among treatments which were similar to the results of Newbold et al. (2004) who reported no change in bacterial N flow, estimated from excretion of urinary PD, when sheep were supplemented daily with 100 mg of mixed EO. Under this study, ELM supplementation tended to improve the N retention and efficiency of microbial N synthesis.
CONCLUSION
Based on this study, it could be concluded that supplementation of ELM could modify the rumen fermentation and has potential as an enhancer for rumen fermentation and methane mitigation. However, further studies using various levels of ELM supplementation in growing trials are recommended.
ACKNOWLEDGMENTS
Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Thailand and the Vietnam International Education Development (VIED), Ministry of Education and Training, Vietnam are gratefully acknowledged for the use of research facilities and financial support, respectively.